Supply Chain Challenges in the Biopharmaceutical Industry: A Case Study Following the 2011 Tsunami in JapanSupply Chain Challenges in the Biopharmaceutical Industry: A Case Study Following the 2011 Tsunami in Japan
https://bioprocessintl.com/wp-content/uploads/2014/09/062014-SupplyChainChallenges.mp3
 Global manufacturing of biopharmaceuticals for human use helps save the lives of millions of people and is a large commitment to public health. The industry operates in an environment with financial uncertainties and complex international supply chains, so the question of risk mitigation is paramount. There is an expectation that comprehensive risk mitigation programs should be in place to minimize the risk of supply chain interruptions that would negatively affect the manufacture of these vital therapeutics. Here we share how a natural disaster affected GE Healthcare Life Sciences’ supply chain and discuss how such a comprehensive risk mitigation program enabled proactive management of the crisis. Finally, we show how supply was maintained to support continued manufacturing of life-preserving therapeutics.
Global manufacturing of biopharmaceuticals for human use helps save the lives of millions of people and is a large commitment to public health. The industry operates in an environment with financial uncertainties and complex international supply chains, so the question of risk mitigation is paramount. There is an expectation that comprehensive risk mitigation programs should be in place to minimize the risk of supply chain interruptions that would negatively affect the manufacture of these vital therapeutics. Here we share how a natural disaster affected GE Healthcare Life Sciences’ supply chain and discuss how such a comprehensive risk mitigation program enabled proactive management of the crisis. Finally, we show how supply was maintained to support continued manufacturing of life-preserving therapeutics.
The Complexity of Manufacturing
Biopharmaceuticals are complex molecules. So the manufacture of consistent, high-quality products over multiple batches is a challenge. Since the launch of Sephadex media over 50 years ago, GE Healthcare Life Sciences has been working in partnership with the industry and has developed extensive expertise supporting all aspects of developing the complex processes required for success. We know that consistent, high-quality end products depend on the use of equally consistent, high-quality key manufacturing components.
Achieving regulatory approval of a manufacturing process is complex, time consuming, and requires identification of all key manufacturing components. Securing multiple sources of key manufacturing components increases drug approval complexity and the time required. As a result, they are often single-sourced, so securing their supply is important. Considering that a biopharmaceutical can have a lifetime of 30 years or more, reliable long-term supply is essential.
Global financial uncertainties and natural disasters — such as the volcanic eruption in Iceland in 2010, the earthquake and tsunami in Japan in 2011, and hurricane Sandy that hit the US east coast in 2012 — highlight just how vulnerable today’s supply chains can be to unforeseen events. And more everyday challenges have the potential to threaten supply chains. Many of today’s more widely adopted business strategies aim to minimize costs and focus on core business. So they involve approaches such as lean manufacturing, just-in-time deliveries, and varying levels of outsourcing. Such strategies also have the potential to stretch a company’s supply chain to its breaking point because they rely so heavily on receiving key components at exactly the right time.
Regulatory authorities prefer pharmaceutical manufacturers to have a full understanding of and control over their supply chain. This further supports the need for a structured, risk-based process for choosing and evaluating service and technology suppliers to support biotherapeutic production processes. The challenge facing such companies is how to manage all potential risks in a complex supply chain that covers many tiers and stretches around the globe. The solution lies in understanding the risks and identifying the pinch points where problems can disrupt your supply chain and then taking appropriate measures to prevent those problems from negatively affecting your business.
Risk Management of the Supply Chain
Risk assessments are an important tool for ensuring product safety, efficacy, consistency, and supply. Many companies in both the United States and the European Union are using ICH Q9 as a basis for identifying the criticality of various components of product safety, efficacy, and supply. For example, raw-material risk assessments require cross-functional input from all departments, including supply, product development, manufacturing, quality control, quality assurance, and other contributors. Having the right expertise over a spectrum of areas is vital for a risk assessment to be meaningful.
Fundamentally important is a continuous supply of product components that meet expected quality standards. To that end, raw-material control strategies play a crucial role. Items to consider when selecting a vendor include the vendor’s in-house quality system, solvency, length of time in business, and geographic area. You should also know whether it supplies multiple industries or just one or two biotherapeutic manufacturers. Strict change-control sections should be included in supplier agreements and cover the criteria that require a vendor to notify you of changes in products or component suppliers.
You also should ask vendors to provide access to their business continuity plans (BCPs). Ideally, those plans will follow international standards and include processes to minimize the probability of an incident occurring. Action plans should minimize the negative impact to both vendors and their customers should incidents occur.
When deciding on a vendor for a single-sourced component of a manufacturing process, you should establish whether a vendor has in place a practical strategy to ensure continuity of supply during the recovery phase of its business continuity plan. Such a strategy provides added reassurance to companies as long as distribution of stock during a supply challenge is actively managed to ensure fair access.
For vendors, it is important that they assess and identify raw materials and suppliers that affect their key products the most. They must also assess revenue drivers, the suppliers at risk of not delivering, and the types of events that could disrupt them. You also need to determine how much time it would take to implement and validate a new supplier of single-sourced raw materials. Based on that assessment, it is possible to start to address pinch points, develop appropriate risk mitigation strategies, and set up controlling action plans. Those tasks also should include setting up legal agreements for supply conditions and audit frequencies. In certain cases, a supplier will allow you to audit its facility — reviewing both security measures and the conditions in place to ensure high safety standards.
Minimizing Supply Chain Interruptions
As a leading supplier of chromatography media, GE Healthcare Life Sciences has a comprehensive strategy in place to help secure a continuous supply of chromatography media used in the manufacture of biopharmaceuticals for human use. Most biopharmaceutical companies have chromatography media single-sourced because of the regulatory constraints discussed previously, a drive for cost effectiveness, and the complexity of having alternative purification solutions. Consequently, over many years of supplying to the biopharmaceutical industry, our company has taken a proactive approach of continuous improvements in all elements of its chromatography media supply chain, covering both production and supply to customers.
GE Healthcare Life Sciences has a long-term investment strategy to continuously improve the safety of its manufacturing facilities and reduce its environmental impact. Specific actions have included infrastructure investments to mitigate risks of fire and explosions, which includes regular training and exercises with local emergency services to test emergency preparedness. The business continuity plan implemented at the Uppsala manufacturing site in Sweden is based upon British Standard BS25999-1 and is continuously audited by external organizations.
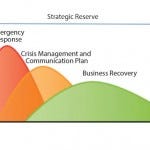
FIGURE 1: GE Healthcare Life Science’s business continuity plan (BCP) for enabling continuous
operation during supply chain challenge
In the event of a serious incident at that manufacturing site, the BCP works in three main phases (Figure 1):
emergency response — aim to minimize the impact of the incident
crisis management — aim to handle the impact including communication to customers
business recovery — aim to restore lost capacity and still serve customers’ needs.
Underpinning this BCP is an on-going comprehensive supply chain management strategy, which places rigorous demands on our company’s suppliers.
Application of a Comprehensive Business Continuity Plan
Despite the strategies to mitigate risk in place at the time, our supply chain was affected by the devastating natural disaster in 2011 where an earthquake and tsunami hit a coastal area of Japan. The impact of this disaster was not only felt locally, but its impact spread around the globe as Japanese-based suppliers’ infrastructure was devastated by the incident and they were unable to supply their global customers. First and foremost the tsunami incident was a tragic human event, but it also served to demonstrate how a local natural disaster affected industries globally and to highlight the vulnerability of today’s global supply chains.
First-Hand Experience
One of our company’s raw-material suppliers used to manufacture a crucial chromatography media, but was severely affected by the 2011 tsunami in Japan. The immediate business impact was a shortage of N,N-methylenebisacrylamid (MBAA), an ingredient in Sephacryl chromatography media, which are used in several approved manufacturing processes for human therapeutics.
As soon as the effect on supply was identified, the comprehensive BCP response plan was activated. The most crucial element of the program was clear: transparent communication to all users of Sephacryl products. This vital communication initiative was led by our CEO, Kieran Murphy, and continued throughout the duration of the incident until normal supply was resumed. The company established a cross-functional task force team, which included members from R&D, management, communication, manufacturing, regulatory support, and customer support as well as regional representatives and supply chain experts. Ongoing status reports on progress with affected customers were shared across the team to enable suitable responses and actions to be taken to support them case by case.
Another element of the BCP was immediate action to evaluate and qualify a new supplier of MBAA to mitigate the risk of discontinuity of supply to customers. Within three weeks, our company identified a potential new supplier, and a project team in R&D was set up to evaluate and verify equivalence between the MBAA from the current supplier and the new intended supplier. Equivalence regarding chemical and physical properties was verified and process validation of the manufacturing of Sephacryl using this alternative supply was performed.
In the meantime, deliveries of remaining inventory of Sephacryl products were actively prioritized to organizations using the media in approved manufacturing processes of biotherapeutics for human use. Prioritization was case by case, taking into account urgency. The order of importance ran from manufacturing of in-market therapeutics to clinical trials to development studies. In addition, material for direct production (column fills) took priority over users building their own safety stocks. Detailed forecasts were requested from customers to facilitate the prioritization of the manufacture of specific types of Sephacryl media. Regular open communication and full transparency of the prioritization process ensured maximum buy-in and support of our user community. The process qualification work for the alternative MBAA supply was finalized and approved within six months of the accident, and normal deliveries of products resumed.
During this incident, no customer was forced to stop production due to a shortage of required chromatography media. The robust comprehensive BCP meant that while the acute situation was managed, work continued to ensure longer term business continuity. The original Japanese supplier of MBAA moved to a new manufacturing site outside the affected area, and validation of MBAA from this new manufacturing site was successfully performed in Spring 2013. Consequently, there are now two fully qualified suppliers of this important raw material, which further supports future business sustainability.
Strategies Vindicated and Lessons Learned
The successful handling of the tsunami-caused threat to our supply chain and the practical robustness of our comprehensive risk-mitigation driven business continuity planning proved the validity of our approach. However, BCP demands constant innovation. Subsequent to this incident, our company has implemented an additional element to our response strategy. To further mitigate risk, the company has established a wide-ranging strategic reserve of chromatography media specifically to cover for potential supply interruptions during the recovery time following a major incident at our manufacturing site or at any of our company’s critical raw-material manufacturers.
The strategic reserve is a security-of-supply commitment to the industry using GE Healthcare Life Sciences media in approved manufacturing processes for human therapeutics and vaccines. The reserve is held in an inventory geographically separated from the regular media production sites. Its content and volumes are reviewed regularly to reflect actual use in biotherapeutic manufacturing.
Further Commitment to the Industry
To complement the strategic reserve, our company also has aggressively pursued a second-supplier program for critical and important raw materials. Identifying second suppliers follows the same model as described above: qualifying the supplier and ensuring appropriate quality systems, product fulfilling required specifications, and financial and production capacity status. Special care is taken to ensure that, when possible, the alternative supplier is not exposed to the risks borne by the preferred supplier. Identification of second suppliers to all possible critical and important raw materials facilitates emergency preparedness. It also requires users to perform only the additional work of verifying/validating that the second source does not affect their manufacturing processes should a disaster occur that impacts the supply chain.
Having such precautions in place does not guarantee the ability to control all future potential supply-chain interruptions. But it does enhance the ability to seamlessly handle potential supply-chain disruptions and help ensure that life-saving biotherapeutics are available for patients in need.
Corresponding author Günter Jagschies is strategic customer relations leader at GE Healthcare Life Sciences and winner of 2012 BioProcess International award “Downstream Thought Leader of the Decade,” Björkgatan 30 SE 75184, Uppsala, Sweden; [email protected]; 46-18-6120000. David Westman is program manager for the chromatography media security of supply program at GE Healthcare Life Sciences. David Raw is head of sourcing at GE Healthcare Life Sciences and responsible for Europe, the Middle East, and Africa.
You May Also Like






