April 2015 Supplement
As this special issue of
BioProcess International
goes to press, an increasing number of cell-based therapies are advancing through preclinical investigation into clinical development and on toward commercialization. Although clinical efficacy will be the primary metric for product approval, the ability to manufacture these therapeutic products consistently, reliably, and cost-effectively will continue to be a key driver and predictor of commercial success.
Several articles presented herein describe major issues and challenges facing developers of cell-based therapies. Although some of the development and manufacturing strategies described depend on whether a therapy is autologous or allogeneic, a few key considerations apply equally to both types of products.
The Importance of Product Understanding
At the top of that list is the dictum, “know thy product.” Developing a thorough understanding of the composition and critical quality attributes of a cell-based product is central to developing a successfu...
Much mystique and mystery surround the emerging industries of cell therapy and regenerative medicine. As companies progress toward commercial manufacture with potential game changers (e.g., cures for cancer and diabetes) the industry could be on the verge of significant breakthroughs. However, with no real successes to date, the question is raised: What core attributes are required to achieve commercial success? The tale of Dendreon’s struggles highlights how difficult it can be to commercialize even an approved cell therapy product.
Since early 2004, my company has worked with organizations dedicated to cell therapy and regenerative medicine to help them develop and implement commercial-scale manufacturing for a wide range of therapies. Our work has helped us identify elements that build a solid foundation for game-changing product development in these fields. We call these elements the “five pillars of success”:
This article (the first of a two-part series) will cover each of the five pillars as a means...
Figure 1 (a)
Early stage harvesting relied on
open manipulation in a laminar hood;
(b)
automatic harvest system performs an entire
harvest process in a controlled environment.
Cell therapy is the injection of cellular material into patients. The injected cell-therapy product (CTP) usually consists of intact living cells. In recent years, cell therapies have evolved and matured, moving from academia to industry. That maturation is reflected in the number of open clinical trials that include the term
cell therapy
in their descriptions: To date, there are more than 8,700 open trials listed on the US National Institutes of Health’s online database (clinicaltrials.gov), most of which are in phase 2 (~4,000) or phase 1 (~2,500). Significantly, more than 1,000 trials are in phase 3, although only a handful of products have matured to clinical approval.
Progress is slow for several reasons. First, cell therapies are highly complex. Opinions still differ over the merits of autologous and allogeneic treatments,...
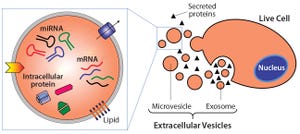
Figure 1:
Extracelluar vesicles are essentially membrane-bound samples of cellular cytosol that transport cargoes such as miRNA, mRNA, and proteins. Exosomes form from fusion of multivesicular bodies with the plasma membrane, whereas microvesicles bud directly from live cells. Apoptotic bodies result from outward blebbing of the cell surface during apoptosis, but are not secreted from healthy cells.
Extracellular vesicles (EVs) are emerging as a potential alternative to some stem-cell–derived therapeutics (
1, 2
). Sometimes called
exosomes,
they are small, secreted vesicles that can possess similar therapeutic mechanisms to whole cells, possibly representing the active pharmaceutical ingredient. In the past 15 years, academic and industry interest in EVs has exponentially increased as mounting evidence demonstrates their role in physiology and pathology as well as their therapeutic potential.
In light of growing efforts in using EVs for research and therapy, optimizing EV manufacturing is important. H...
Maximizing PMBC Recovery and Viability: A Method to Optimize and Streamline Peripheral Blood Mononuclear Cell Isolation, Cryopreservation, and ThawingMaximizing PMBC Recovery and Viability: A Method to Optimize and Streamline Peripheral Blood Mononuclear Cell Isolation, Cryopreservation, and Thawing
The quality of peripheral blood mononuclear cells (PBMCs) isolated from whole blood has a significant impact on their subsequent analysis. Maximizing recovery, viability, and functionality of isolated PBMCs is essential to the reliability and consistency of downstream applications, particularly within cell therapy manufacturing.
The standard method for purification of PBMCs is density-gradient centrifugation. It requires precise layering of whole blood over a density medium (e.g., Ficoll polysaccharide reagent from GE Healthcare), with careful pipetting of the floating cell layer after centrifugation. Analysts must take meticulous care throughout the procedure to keep from mixing layers before and after centrifugation. Newly available SepMate tubes from Stemcell Technologies can greatly reduce the time for optimal PBMC isolation from whole blood. But implementing them would require validation efforts to ensure that they perform equivalently to traditional Ficoll methods.
Optimizing PBMC isolation methods ...
Figure 1:
The 21st-century pharmacopeia, including the convergence of “regenerative medicine” therapeutic approaches
Cell-based immunotherapies (iTx) are emerging as a truly transformative therapeutic modality that is both complementary and convergent with existing regenerative medicine approaches, including gene therapy, cell therapy, and tissue engineering (Figure 1). Critically, iTx offer step-change improvements in efficacy compared with current standards of care (
1
) for a range of clinical indications and unmet therapeutic needs — particularly oncology.
The clear efficacy of iTx is in contrast with some previous regenerative medicine approaches, including early mesenchymal stem cell (MSC) therapies for articular chrondral repair, which demonstrated only equivocal efficacy (
2
) despite a substantial increase in cost as compared with existing standards of care (
3
). Therefore, although clinical adoption (
4
) and pharmaceutical engagement in regenerative medicine may have been limited to date (
5,...
https://bioprocessintl.com/wp-content/uploads/2015/04/McIntyreSuman-VV-FINAL1.mp3
Technology transfer moves a process into a different environment and as a result, the
product should be properly defined before the transfer is initiated. Before technology transfer, raw
biological material collection (e.g., bone marrow, apheresis) and manufacture may be colocated,
typically at a clinical site (a). Following technology transfer, clinical and manufacturing sites are no
longer in close proximity (b).
Cell therapies offer enormous promise for treatment of a range of conditions by replacing damaged tissue or leveraging the body’s own resources to heal itself. Not surprisingly, the cell therapy industry is growing rapidly and is poised to have a major impact on healthcare and disease treatment. The Alliance for Regenerative Medicine (ARM) has reported on the robust state of the industry, noting that revenue from cell-derived products grew from US$460 million in 2010 to $1.3 billion in 2013 (1).
A critical aspect ...