Foundation Elements for Cell Therapy Smart ScalingFoundation Elements for Cell Therapy Smart Scaling

Figure 1 (a) Early stage harvesting relied on
open manipulation in a laminar hood; (b)
automatic harvest system performs an entire
harvest process in a controlled environment.
Cell therapy is the injection of cellular material into patients. The injected cell-therapy product (CTP) usually consists of intact living cells. In recent years, cell therapies have evolved and matured, moving from academia to industry. That maturation is reflected in the number of open clinical trials that include the term cell therapy in their descriptions: To date, there are more than 8,700 open trials listed on the US National Institutes of Health’s online database (clinicaltrials.gov), most of which are in phase 2 (~4,000) or phase 1 (~2,500). Significantly, more than 1,000 trials are in phase 3, although only a handful of products have matured to clinical approval.
Progress is slow for several reasons. First, cell therapies are highly complex. Opinions still differ over the merits of autologous and allogeneic treatments, different tissue sources for stem cells (e.g., embryonic, adult bone marrow, umbilical cord, placenta, and adipose tissues), and the types of cells for development (e.g., hematopoietic, mesenchymal stromal, or progenitor). Second is a lack of mature regulatory guidelines and predicate approvals from bodies such as the US Food and Drug Administration (FDA) (1, 2). Finally, challenges in scaling manufacturing also limit the number of late-phase clinical trials and approvals so far.
The unique difficulties in scaling up production of CTPs has to do with their nature as both pharmaceuticals and living cells. Pharmaceuticals are regulated products, for which strict guidelines and limitations for process changes ensure quality and safety in scaled-up production. Changes require studies to prove that they do not alter a product’s critical attributes (1, 2). Living cells, however, react to their growth environments by modifying their protein expression profiles, viability, and other characteristics. Any change in culture conditions — e.g., media formulation, serum concentration and replacements, duration of processing, adherent-surface materials and their structure, and shear forces generated when cells are moved from culture dishes to bioreactors — will change cell phenotypes. If such critical attributes change, then so may their mechanisms of action (MoA), efficacy, and even safety as products. Such changes can influence their performance in clinical trials. So consistent culture conditions are needed to produce reliable and reproducible data about a CTP.
If changes occur in cell production methods during later-stage clinical studies or after commercialization, comparability studies would have to prove that those process modifications did not affect the safety or efficacy of a given product and that earlier trial results are relevant to later studies. It would be difficult to prove comparability at that point without additional clinical trials. Therefore, progressing through clinical trials without first developing and maturing a cell therapy manufacturing process might be cost effective initially. But it could create significant financial burdens and risks later on when a company must prove comparability for cells made using early, small-scale production methods with cells produced by industrial culturing technologies such as bioreactors.
Critical Foundation Elements
Naturally, a culture process will evolve as a product matures through its clinical path. It is neither expected nor even feasible to fully develop or scale up a process at early clinical stages. Nevertheless, to allow for scalability and maturation of a process in later stages, several basic foundation elements must be considered and integrated at the beginning that can facilitate scaling of the process later on. Here we address those basic principles and present case studies of implementing them in early stage development.
Foundation Elements for Products: As with any project, the first step in developing a scaled-up cell therapy process is planning and defining the foundation elements of its product. The first element is defining expected target product attributes — referred to in the pharmaceutical industry as a target product profile (TPP). Ideally, the TPP describes how a product will be used. For the manufacturer, the TPP helps identify project goals and potential risks. It can serve as a basis for discussions among different groups within the company (e.g., clinical, development, drug safety, manufacturing, marketing, regulatory, research, and quality) before and during development. This profile assists them in understanding the cell therapy manufacturing scale needed, the cell storage and delivery system, and even a price point (3). That information allows a developer to design its cell therapy manufacturing process to support expected needs and define critical milestones and goals along the way.
The second product foundation element is characterization. Such knowledge must be related to the proposed mechanism of action (MoA) and define the CTP’s critical quality attributes (CQAs). A CQA is a physical, chemical, biological, or microbiological property or characteristic that must be within an appropriate limit, range, or distribution to ensure desired product quality (4). Once the basic CQAs are defined, a company must make an effort to develop reliable analytical tools for measuring those attributes. Such tools do not only include biological and characterization analytics, but they also should include process analytical tools that can help a company analyze and control manufacturing through timely measurements (during processing) of critical quality and performance attributes for raw and in-process materials and processes. The goal is to ensure final-product quality (5).
Once a cell-therapy product’s TPP is better defined, and known characteristics and quality attributes are associated with reliable analytics, the sponsor company can start to design an ongoing project for process development.
Foundation Elements for Processes: A CTP process should be built on several basic foundation elements, which include scalability, closed systems, automation, environmental control, streamlining, and regulatory compliance.
Scalability: All technology components or basic technologies must be scalable to meet the needs of the TPP. There is no need to develop a cell therapy manufacturing process at the final scale initially, but the chosen technology must be scalable to meet that need later on. In some cases, scale-out could be considered as scalable: using a few devices in parallel rather than one large device. We highly recommend finding a technology platform that not only fits the need for the scale to be used, but that also can be scalable without changing its basic technological principles to meet market needs.
Closed Systems: Because CTPs cannot be sterilized at the end of manufacturing, it is critical to ensure their sterility through aseptic processing and monitoring instead. Open manipulations add a major risk of contamination and endangers a product. The risk increases further downstream because of a reduced overall ability to detect and neutralize contamination.
Automation: Many industries regard automation as an operational advantage. In CTP manufacturing, it helps ensure product consistency and process control. As cells change their phenotype in response to environmental changes, consistency becomes critical. Automation can reduce human error and lower the number of operators needed to run a process — thereby reducing contamination risk.
Environmental Control: Cell culture is a dynamic environment containing living cells, so current good manufacturing practices (CGMPs) require control of a culture process and its conditions. Controlling and monitoring process parameters and trends increases production-line reliability, resulting in safer products and improved CTP efficiency down the road.
Streamlining: Cells that are held outside their natural environment (in this case, away from culture conditions) will change, could be damaged, or might even die. Streamlining the cell therapy manufacturing process to minimalize hold duration is critical. It is important to define critical hold times and the longest duration that will not adversely affect a CTP’s phenotype or viability. This is even more critical as a product moves downstream toward its final formulation.
CGMP compliance: Broadly defined, regulatory compliance covers materials used, work flows, training, process reliability, software, and documentation.
Our Process Overview
Pluristem Therapeutics manufactures placenta-derived mesenchymal stem cells (MSCs) for a number of indications. The average dose used in our clinical trials is several hundred million cells. Once market authorization is reached, there will be a commercial need for batch sizes that can average several hundred billion cells. To prepare for such capacities, we integrated the scale-up foundation elements listed above into our manufacturing process between phases 1 and 2. The process steps are as follows:
Isolation and expansion of cells from placenta
Cryopreservation and full characterization of cells at passage 3
Thawing of a single vial of cells for further culturing and expansion in a proprietary bioreactor system
Downstream processing and final formulation.
The main goal of this development stage — termed internally as manufacturing process 3 (MP3) — is to design a process that manufactures high-quality cells and integrates the aforementioned foundation elements without changing critical processes that might affect those cells’ CQAs. Below we describe a few critical steps that were optimized in a new process, each one serving as a case study for several scale-up foundation elements. We focus here only on the second stage of culturing, which begins at the bioreactor.
Bioreactor Growth and Harvest
Foundation elements involved here include scalability, environmental control and CGMP compliance, closed systems, and automation.
Scalability with a Fibra-Cel Matrix: Adherent cells such as MSCs are cultured on treated plastic surfaces to which they must adhere to grow. Small-scale culturing in laboratories uses culture dishes with an average surface area of 75 cm2, with a typical yield of 1–2 million cells/dish (6, 8). An average MSC harvest density is ~25,000 cells/cm2. As mentioned above, cell-therapy doses can range from a few million to over 500 million cells. Manufacturing on such a scale approaches the limit of capacity for current technologies such as larger culture tray units (6, 8). To overcome the limitations of two-dimensional (2D) culture, Pluristem chose to use Eppendorf’s scalable Fibra-Cel matrix as a microcarrier system for three-dimensional (3D) culture in perfused-bed bioreactors (Eppendorf 5-L vessels with a T700 control system from Finesse Solutions). Our MSCs adhere to Fibra-Cel fibers, with a high surface-to-mass ratio (>1,000 cm2 or 25 × 109 cells per gram of Fibra-Cel matrix) within the bioreactor’s 3D environment for efficiency and maximized cell yields.
Cell growth on this platform can be summarized as follows: A harvested 2D cell suspension is seeded into the bioreactor at a known and constant concentration and expanded there under controlled environmental conditions (temperature, pH, dissolved oxygen (DO), and nutrient concentration — with fresh media perfused to maintain the latter at steady-state levels). Once cells reach a desired density, they are harvested from the matrix by means of enzymatic dissociation and mechanical force.
Our 3D platform offers many advantages over 2D platforms. It is more cost effective, requires fewer operators, and occupies a much smaller footprint (8). It also provides good repeatability and greater control over a number of growth parameters. All those advantages contribute to the system’s inherent scalability, as well. The Fibra-Cel system has delivered consistency with 30, 100, and 400 g of matrix, showing that it can be linearly scaled up to harvest high-quality cells without changing product CQAs as long as culture conditions are controlled.
Based on our data, we are confident that this system can be scaled up even more in the future, although we are also prepared for a scale-out option if needed. The culture process was designed such that each run is built on more than one bioreactor (running in parallel) from the same initial vial. We can pool together the outputs of several bioreactors if necessary. But that is only because we have collected extensive data on culture conditions since our early stages and accumulated the necessary experience to ensure that pooled cells meet our defined TPP.
Environmental Control and CGMP Compliance with a Finesse/DeltaV Control System: A bioreactor control system’s primary function is to maintain defined environmental conditions (e.g., temperature, pH, DO levels, and perfusion rates). However, certain additional features should be considered early on because they are likely to become necessary when implementing the control system for large-scale production. Modularity is the ability to maintain those environmental conditions over a broad range of reactor sizes while controlling a large number of reactors through a central control unit. Extensive data logging for a CGMP growth process should produce a detailed record of the entire growth period and sound an alarm if anything goes wrong. Customizable software could be tuned to make a process work at all scales. And materials will need to be used in ISO007-level cleanrooms.
During our transition to MP3, we chose to use a T700 SUB–DV control system from Finesse Solutions because it met the requirements listed above. This control system is based on Emerson Process Management’s DeltaV software. It is CGMP compliant and scalable, with compliant data logging and reporting. Furthermore, the system is customizable, has redundant controls and ports, and is built and designed for cleanrooms. Working together with the vendor, we designed and validated a perfusion algorithm to fit our needs. The resulting system provides on-line monitoring and control through a secure and validated interface with alarm systems that detail specific errors. For example, an alarm is raised when system pH crosses its upper limit or the medium feeding perfusion drops below its lower limit. That gives us powerful control of our culture system, with the ability to intervene if failures occur. System implementation required tuning and adjusting temperature, pH, and DO cascades, then optimizing the proportional-integral-derivative (PID) controller parameters to reach a level of control that is similar to what we achieved with our previous control systems.
Closed Systems and Automation Using an Automatic Harvest System: Harvesting adherent cells from Fibra- Cel disks requires a combination of enzymatic digestion and agitation. During our phase 1 early stage manufacturing processes, agitation was manual and required opening bioreactors to release the microcarriers (Figure 1a). That early stage harvest process was unsuitable for scale-up because we needed a streamlined, automatic system that would eliminate open manipulations to reduce the risk of contamination, shorten process duration, and provide better reproducibility and control. So we developed a new 3D culture harvest system (Figure 1b) that fills and drains a bioreactor with relevant solutions and agitates a microcarrier basket to release adherent cells. Once those cells are released from the Fibra-Cel disks, the bioreactor is drained and cells are collected into a sterile bag. To reduce operational errors and improve reproducibility, a control system automatically performs those steps and logs all relevant data.
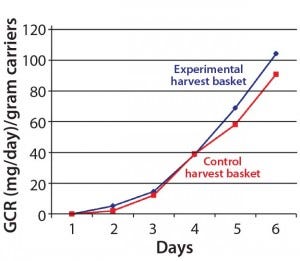
Figure 2: The new harvest basket did not
affect cells’ glucose consumption rate (GCR).
The original microcarrier basket had to be configured to fit our new harvest system and allow agitation of the packed bed of disks during harvesting inside the reactor vessel itself (to maintain sterility). Following that configuration, we tested the uniformity of cells grown on microcarriers in the newly designed basket and found them to be appropriate and comparable to those from a control basket. No significant differences were found among different areas in the new basket. Cell growth rates — determined by measuring glucose consumption rate (GCR) — were similar in both the new and original basket configuration (Figure 2).
Postharvest Cell Concentration and Washing
Foundation elements involved here include closed systems, automation/ streamlining, and scalability. Once cells are harvested from a bioreactor, downstream processing begins. Harvested cells come in a suspension that includes residual growth media, serum, and enzymes that dissociated the cells from the microcarriers. That is not an ideal environment for the cells because it provides no nutrition and conditions our suitable culture conditions (oustide the bioreactor and its controls). So they need to continue into downstream processing as soon as possible. Essential cell washing and concentration is performed through repeatable steps intended to remove media and residuals, then reformulate the suspension for cryopreservation and injection. When a desired purification level is achieved, cells are diluted to the dosage concentration before moving forward to final formulation.
In our early phase 1 manufacturing process, we washed and concentrated cells using batch pellet centrifugation in 500-mL centrifuge tubes. Supernatant was discarded, and cell pellets were resuspended in suspension media through manual pipetting. That process was repeated several times, with cells centrifuged at high speed for 10 minutes during each cycle. Each CTP lot required 10–20 centrifuge tubes to undergo that centrifugation process, which made the process time-consuming and demanded very skilled manual labor. A centrifugation process could be very stressful to cells, and they can aggregate in pellet form. The process of washing and concentrating cells included many open manipulations, which increased the risk of contamination to the final product.
Scalability presents another problem for batch centrifugation. In a scaled-up process for a reasonable phase 2 trial, the final volume that needed to be concentrated was large: ~16–20 L of cell product suspension. That required 40 500-mL centrifuge tubes to be washed and concentrated several times each. The time needed to perform such a process would have increased to a critical level that could harm a CTP and alter cell viability and biological activity.
For those reasons, our goal was to find a way of concentrating and washing cells that would be less stressful to them, less time consuming, and could be performed using a closed system. Not only would that provide for efficient mass-production of CTPs, but it would also help our process meet CGMP standards, which include the need for aseptic procedures in closed systems with controlled processes.
A few technologies meet the need for aseptic cell centrifugation. We chose kSep technology from KBI for three main reasons: scalability, automation, and closed manipulation. This technology operates on a principle of continuous centrifugation. Cells are pumped continuously into one of four chambers that rotate at high speed, creating two opposing forces: the forward flow of cell suspension or media and the opposing centrifugal force. Through a balance of centrifugal and fluid-flow forces, the instrument retains cells as a concentrated fluidized bed under continuous flow. That allows for washing and concentrating cells using a closed and automated system. Another important benefit of this technology is that keeping the cells in suspension throughout the process maintains them in a more natural environment with lower shear forces than would be found in typical centrifugation.
Closed System: Eliminating open manipulation was the biggest challenge we encountered. The kSep system uses a simple single-use kit that is assembled from two sterile parts connected to one another using a welding machine. The kit tubing is made of C-Flex plastic from Saint-Gobain Performance Plastics, which allows for closed aseptic welding of all essential materials needed: the cell suspension bag, washing and priming media, the centrifuge tube for collection of harvested cells, and a waste container.
Automation and Streamlining: Our harvest system is fully automated, can be operated by only one person, and offers an option of manual operation. The program runs a sequence of concentration, washing, and harvest steps based on user-defined parameters such as centrifuge speed, flow rate, duration, and harvest volume. Based on our needs, we built a process that will be suitable for our cells and that is short (~50 minutes each run), safe, and simple.
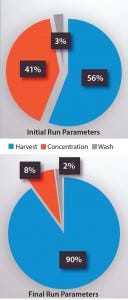
Figure 3: Run parameters include centrifuge
speed, flow rate/duration, and harvest volume.
On the top, 44% of the cells were lost during
concentration and washing with the initial run
parameters. On the bottom, that loss was
reduced to 10% by optimized parameters.
The biggest development challenge was to optimize parameters for placental-derived PLX cells (with optimization defined as choosing parameters that yield the highest cell recovery). We did so by performing a series of runs to test a range of parameters for each step. For success criteria, we chose a cut-off of >80% recovery for the process and tested different parameters until we achieved that. As those parameters were determined, we also tested the effects of continuous centrifugation on cell quality and biological activity (Figure 3).
Scalability: Although most single-use systems are not scalable to larger volumes (10–200 L), they can be scaled-out with the use of multiple units. But the kSep system is fully scalable. There is no limit to the volume of cell suspension it can process in one run; there is only a maximum number of cells it can concentrate in one cycle. To evaluate that, we tested the capacity of the centrifuge by performing a series of experiments to determine minimal and maximal numbers of cells that could be processed in each run. We tested combinations of one, two, and four chambers and found that each chamber had a minimum cell number of 1.15 × 109 PLX cells and a maximum of ~10 × 109 PLX cells (40 × 109 cells for all four chambers) — an impressive capacity. With a per‑chamber flow rate of 0.15 L/min, a 64-L cell suspension can be concentrated in about two hours. When further scale-up is necessary, the kSep system has a built-in ability to concentrate up to its full capacity, harvest, and immediately proceed to a new cycle with a new container of cell suspension.
Final CTP Formulation
Foundation elements involved here include closed systems, automation, and scalability. After concentration and washing, concentrated cells are counted and then diluted with a suspension solution to twice their final concentration. They are counted again to verify accuracy, after which they are diluted 1:1 with a freezing solution to produce the final cell formulation (generally 10 or 20 million cells/mL).
In early stage manufacturing, final formulated CTPs were filled into 50-mL freezing bags, followed by cryopreservation and storage. The process was performed manually, which had several drawbacks: Open manipulation greatly increased the risk of contamination, and manual filling carried with it the potential for inaccuracy and human error. Additionally, the freezing bags we used were somewhat cumbersome. They required a minimum volume of 10 mL and a maximum volume of 30 mL, which both limited our flexibility in adjusting dose volumes and forced us to store multiple doses in a single container. The storage and shipping process required specialized cassettes and racks, which risked bag breakage.
Working with such freezing bags also presented difficulties for end users because each bag contained a large volume of CTP that was not necessarily suitable for clinical site use, so overall work with the bags was inconvenient for clinical staff members. We received a few reports of bags breaking during the thawing process at some clinical sites. Because it is more acceptable to work with rubber-stoppered vials at clinical sites, we modified our filling process to use Crystal vials from Aseptic Technologies.
Closed System: Because formulation–filling is the final step in the CTP manufacturing process, reducing the risk of contamination is of utmost importance. Product contamination at the point of vial filling would disqualify an entire batch, which would in turn represent a financial loss of serious significance. Furthermore, detecting a contamination at this stage is difficult because sterility testing is performed only on samples. Undetected contamination could risk patient health.
Aseptic Technologies designed a presealed vial system in which filling takes place inside a laminar-flow hood, so the entire process is closed following solution formulation. A disposable needle pierces the septum of a sterile vial and accurately dispenses a final PCT formulation at a desired volume. The septum is made of self-sealing rubber, which closes up the hole as soon as the needle is withdrawn. The septum then passes through a lasing machine to create a true hermetic seal. After lasing, a protective cap is installed over the septum to protect it, a step that is performed under sterile conditions, with care taken not to directly touch the septum.
Sealed and impenetrable vials (because of both the lasing process and the protective cap) are very advantageous in terms of storage and shipping to clinical sites. They ensure CTP sterility all the way to the point of injection.
Automation: An automatic system becomes especially useful when scale-up demands filling of more vials. Filling large numbers of vials by open manipulation takes more time and increases the risks of contamination and uneven volume distribution.

Figure 4: (left) the M1 filling system; (right) a close-up view of the dispensing needle
Although not fully automatic (requiring an operator to insert, fill, lase, and cap the vials), the Aseptic Technologies M1 filling system we chose for this scale represents significantly more automation than our previous process did. We can fill three vials per minute now, and by working with two operators (one filling vials and one who lases and caps the vials) we can streamline the process (Figure 4).
The M1 system contains a peristaltic pump that transfers precise volumes of final CTP formulation from a spinner flask. The pump can be calibrated at the beginning of the process and during the process if an operator switches filling volumes. Moving to automated pump filling allowed us to fill vials with high precision and good repeatability, which ultimately prevents wasted volume and homogenizes vials. The density of the cell formulation must be defined for this pumping step to be precise.
For filling and storage of our product, we chose 6-mL Crystal vials. In addition to the volume flexibility those provide, physicians are already familiar with using rubber-stoppered vials for injections. Thawing vials is considerably easier than thawing bags. Such point-of-use considerations may seem nonessential at the process-development stage, but they can in fact contribute greatly to a product’s ultimate success and should be part of its TPP.

Figure 5: Internally developed vial box can be used for cryopreservation, storage, and shipping.
We have developed a custom vial box for cryopreserving and storing Crystal vials (Figure 5). Because no available boxes were compatible with gaseous nitrogen storage, we had to develop a storage box that would provide for even temperature distribution during cryopreservation and storage as well as protecting vials during transport. This custom box was another reason for filling all sample volumes in 6-mL vials.
Scalability: Because the technology and process already exists, we can scale out manufacturing by simply adding another filling system to output more vials. That would mean attaching two filling systems in parallel, thereby halving the time required for the same output. Speeding up the filling process thusly would reduce the total length of time during which cells are exposed to final-formulation solutions and before cryopreservation. That critical parameter should be kept to a minimum.
Another option for scaling is the Crystal L1 robot line (also from Aseptic Technologies), a fully automatic system that can fill 600 vials/hour. It requires an operator only to load a tray of vials and solutions; the rest of the filling process is accomplished through robotic manipulation. This system is completely closed (including its filling needle, unlike the M1 system) and therefore fully aseptic. Because our infrastructure is ready and proven to be safe both for our product and the Crystal vials, transitioning to the robotic system would require minimal adaptation and involve minimal risk to our product — thanks to the similar filling method in both systems.
Begin with the End in Mind
CTP process development is a key part of overall cell therapy research and development (R&D). The natural course of development is slow. Although it isn’t feasible to start with large-scale processing, foundation elements of scale nonetheless must be integrated in early stage development to ensure later-stage success. Identifying those elements begins with defining a product according to its TPP, identifying CQAs, and implementing basic process elements in early stages. Those are likely to include scalable technologies, a controlled and reproducible culturing environment, closed procedures, streamlining, automation, and CGMP compliance.
References
1 OCTMA. Guidance for FDA Reviewers and Sponsors: Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs). US Food and Drug Administration: Rockville, MD, 2008.
2 CBER. Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy. US Food and Drug Administration: Rockville, MD, 1998.
3 Lambert W. Considerations in Developing a Target Product Profile for Parenteral Pharmaceutical Products. AAPS PharmSciTech 11(3) 2010.
4 ICH Q8(R2). Pharmaceutical Development. US Fed. Reg. 71(98) 2009.
5 CDER/CVM/ORA. Guidance for Industry: PAT — a Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. US Food and Drug Administration: Rockville, MD, September 2004; www.fda.gov/downloads/Drugs/ Guidances/ucm070305.pdf.
6 Rowley J, et al. Meeting Lot Size Challenges of Manufacturing Adherent Cells for Therapy. BioProcess Int. 10(3) 2012: S16–S22.
7 Shaw R. Business Model Considerations for Development of Cell Therapies. BioProcess Int. 12(4) 2014: 10–14.
8 Karnieli O. Cell Therapy: Early Process Development and Optimization of the Manufacturing Process Are Critical to Ensure Viability of the Product, Quality, Consistency and Cost Efficiency. J. Commerc. Biotechnol. 21(1) 2015.
For Further Reading
Rathore AS, Winkle H. Quality By Design for Biopharmaceuticals. Nat. Biotechnol. 27(1) 2009. •
Yonatan Levinson, Yonatan Eylon, Anna Heymann, and Anna Zaretsky-Rits are process development engineers, and Ohad Karnieli is vice president of technology and manufacturing at Pluristem Therapeutics Inc., MATAM Advanced Technology Park Building #5, #20, Haifa 31905, Israel; 972-74- 710-8600, fax 972-74-710-8765; [email protected].
You May Also Like






