Artist conception of T cells attacking a cancer cell (www.istockphoto.com)
Just about anyone in the biopharmaceutical industry will tell you that cost is now the primary concern in cell and gene therapy development. It hasn’t even been a decade since “manufacturability” was the main issue at hand — and cost has risen organically from related discussions. Regenerative medicine evolved from medical research rather than from drug-development companies, and technologies that worked in clinical settings haven’t translated directly to manufacturing facilities. Cost is often the problem. Early product successes (that ultimately weren’t so much) made the importance of cost control glaringly clear. And whereas the classical drug field increasingly is called out for massive mark-ups and huge advertising budgets, the price of regenerative medicines truly is linked directly to the cost of making them.
Some experts rightly have pointed out that it’s not fair to compare the cost of a one-time cure (e.g., for cancer) wi...
Regenerative medicine holds great potential for human disease management, with hundreds of cell and gene therapy (CGT) products for tissue/organ reconstitution or replacement in different stages of development and clinical testing for toxicity, safety, and efficacy. For example, currently more than 60 CGTs have marketing authorization (although many with only conditional approval) from central regulatory agencies worldwide (
1
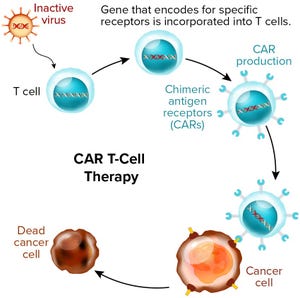
). Those products are treating conditions such as hematopoietic malignancies, immunological disorders, and cartilage disorders. Most of those treatments use culture-expanded autologous or allogeneic cells — some of which are genetically modified, such as for chimeric antigen receptor (CAR) T-cell gene-therapy products.
Nevertheless, the tangible clinical promise of cell, gene, and tissue therapies also has brought about the emergence of tagalong marketing for services both legitimate and dubious, including speculative private cell bank services. By contrast with cord-blood banking (...

 +2
+2Figure 1: Chimeric antigen receptor (CAR) T-cell therapies also are gene therapies. (www.istockphoto.com)
Before we had the 21 CFR 1271 regulation for tissue therapies, the US Food and Drug Administration (FDA) had determined that regenerative medicine was exceptional enough to warrant its own regulations for good manufacturing practice (GMP). Since 2001, the tissue industry has adapted to those new rules while the FDA stepped up enforcement over time. When a cell or tissue product is regulated under 21 CFR 1271, its specific regulations apply before the general regulations for biologics and drugs. But what about combined gene and cell therapies? Are they covered under existing regulations and guidances, or does this new product class warrant its own GMPs within the existing framework for drugs, biologics, and tissue products?
Talk has circulated recently about the possible need for separate regulations for cell and gene therapies. But I do not think that we need separate GMPs for this product class. Gene...
Artist’s conception of the moment a
CAR T cell identifies a cancer cell. (www.istockphoto.com)
As cell therapies move through the clinic toward commercialization, respondents to an Informa Connect industry survey are beginning to look to allogeneic — or off-the-shelf — products as “the next big thing.” Almost 200 people contributed to the
Cell Therapy Analytics Report
, revealing their current positions within the burgeoning cell and gene therapy space and offering their thoughts and predictions for the future.
Most survey respondents work within companies developing oncology products. Of those, the largest group (41%) said that they are developing chimeric antigen receptor (CAR) T-cell therapies specifically. This developmental divulgence reflects the regulatory successes of Novartis’s Kymriah (tisagenlecleucel) and Kite/Gilead’s Yescarta (axicabtagene ciloleucel), both of which shook the drug world when they received approvals in 2017.
Both those products are made using patients’ own cells, which are g...

schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)





