September 2019 Featured Report
Image Credit: https://stock.adobe.com
Every biomanufacturing process begins with transfection of recombinant genes into pools of cells — followed by a succession of screenings from which will emerge (ideally) a single progenitor cell of the new production cell line. Cast aside will be those cells that do not uptake the correct genetic material, those incapable of thriving in bioprocess conditions, those that fail to produce recombinant protein at relevant levels, and those without demonstrated clonality and relative genetic stability.
Over the past several years, advancing technologies for genetic engineering, screening, clone selection, analysis, and data management have brought about a revolution in cell line development. Audiences at the BioProcess International (BPI) conferences have watched this field evolve as presenters shared their insights and reported on their experiences. This year’s BPI Europe and BPI West events continued in that vein with presentations on technology platforms, clonality, cel...
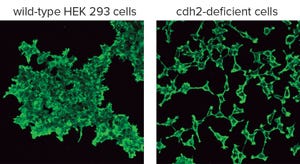
Figure 1: HEK293 wild-type and cdh2-deficient cells were stained with fluorescein-conjugated phalloidin (2).
Cultured cell lines have a diverse range of applications. They are used broadly by cell biologists, clinicians, tissue engineers, biotechnology scientists, and bioengineers. The most important uses of cell culture are in the cell-based assays and production of biologically active recombinant proteins. In recent years, genome editing has been used widely to study the structure, function, and localization of endogenous proteins in cultured cells. However, applying the same genome editing techniques to cell lines also could improve the propagation of cells and help scientists devise novel assays for discovering bioactive molecules. Below, using a cadherin adhesion molecule as an example, I explain a simple approach to genome editing for generating novel cell lines to achieve those goals.
Case Study
Cadherins are cell-surface transmembrane proteins that mediate cell-to-cell adhesion in animals. In 1987...
Photo Credit: https://stock.adobe.com
Monoclonal antibodies (MAbs) have radically transformed the treatment of many chronic diseases, mainly in the fields of oncology and autoimmunity. The overwhelming majority of therapeutic MAbs are manufactured from recombinant Chinese hamster ovary (CHO) cell lines. The original CHO cell line was isolated in the 1950s, and since the early 1980s, it has become the workhorse of the biopharmaceutical industry.
The CHO-DG44 strain was generated after several rounds of mutagenesis that deleted both copies of dihydrofolate reductase (dhfr) genes by ionizing radiation (
1, 2
). The DHFR enzyme catalyzes reduction of dihydrofolic acid to tetrahydrofolic acid, making it central to the biosynthesis of purines, thymidylic acid, and certain amino acids (
3, 4
). For that reason, CHO dhfr–/– strains require supplementation of hypoxanthine and thymidine (HT) in their cell culture media. Several MAbs currently are produced by recombinant CHO-DG44 cell lines to allow for consecutive ...