- Sponsored Content
Manufacturing Challenges of Therapeutic Antibody–Drug ConjugatesManufacturing Challenges of Therapeutic Antibody–Drug Conjugates
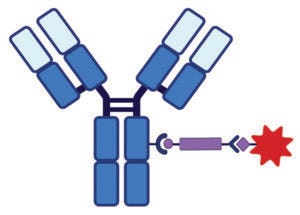
Figure 1A: Antibody–drug conjugate (ADC) structure and delivery pathways; linker chemistry determines the mechanism by which cytotoxin release occurs. This schematic shows a monoclonal antibody (MAb) conjugated to a linker (purple bar)–cytotoxin (red star) combination. Conjugation chemistry typically involves lysine or cysteine amino acids on the MAb.
The pharmaceutical industry has come a long way since 1907, when Paul Ehrlich coined the term “magic bullet” for treatments that could target disease-causing cells while sparing normal, healthy cells (1, 2). Researchers began investigating targeted cytotoxins as therapeutic bioconjugates in the late 1980s. Groups led by Ellen Vitetta at the University of Texas Southwestern Medical Center (3), Ira Pastan at the US National Institutes of Health (4), and John R. Murphy at Boston University (5) fused truncated Pseudomonas exotoxin (PE) and diphtheria toxin (DT) to single-chain monoclonal antibody (MAb) fragments and peptide hormones such as transforming growth factor alpha (TGF-α) and interleukin 2 (IL-2). Vitetta’s work led to a phase 1 dose-escalation trial in refractory B-cell lymphoma patients with an anti-CD22 Fab’ MAb fragment conjugated to a deglycosylated ricin A chain (Fab’–dgA), which showed partial responses in over 50% of patients with >50% CD22+ tumor cells (6).
Pastan’s and Murphy’s efforts led to US Food and Drug Administration (FDA) approval of Lumoxiti (moxetumomab pasudotox-tdfk), a CD-22–targeted PE immunotoxin for treatment of hairy-cell leukemia (7), and Ontak (denileukin diftitox), an IL-2–targeted DT treatment for cutaneous T-cell lymphoma (8), respectively. In the four decades since Vitetta, Pastan, and Murphy launched targeted cytotoxins as therapeutic bioconjugates for cancer, the technology has advanced significantly, and several more products have received market approval — all with antibodies as their targeting moieties. Initially referred to as immunotoxins, these therapeutic bioconjugates are now more commonly called antibody–drug conjugates (ADCs).
Recent clinical success has fueled much excitement about ADCs in the biopharmaceutical industry, and the segment is expanding rapidly. As of 2019, four ADCs had received market approvals in Europe and the United States, and at least 60 more products are in clinical development (9). The number of FDA approvals for ADCs increased to 11 as of September 2021 (10) — all for oncology indications. Considering that ADC development has been extensively reviewed (9, 11–13), here we offer a brief update on the latest advances and challenges in the design, construction, and manufacture of these therapeutics.
Structure and Function
Mechanism of action drives product design for an ADC based on its three components: a targeting moiety, a “payload” that is typically a highly active cytotoxin, and a chemical linker to join those two. ADCs are designed to bind to cell-surface receptors and get internalized through receptor-mediated endocytosis (RME), then trafficked into endosomes and ultimately lysosomes (Figure 1b). Following internalization of the ADC construct, ADC–antigen complexes are fused with endosomes, which break them up for receptor recycling and transport ADCs to lysosomes. Finally, an ADC molecule undergoes lysosomal degradation through a number of pathways to release its cytotoxin, which then binds to its target (14).
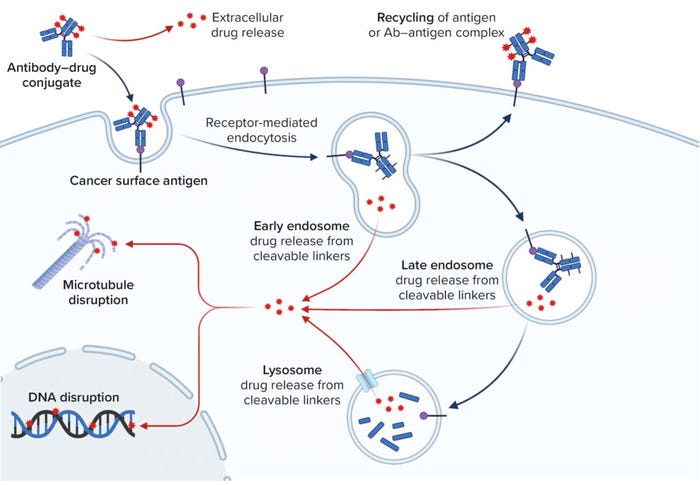
Figure 1B: Antibody–drug conjugate (ADC) structure and delivery pathways; this schematic illustrates the pathway an ADC takes once it binds to an antigen on a target cell. Binding at the cell membrane results in endocytosis of the ADC, which is trafficked to an endosome or lysosome, where the cytotoxic payload is released. The mechanism of payload release depends upon the design of the linker–toxin (LT) moiety. The intracellular target of the payload is usually disruption of microtubule function or DNA replication.
Monoclonal Antibodies for Targeting: The ADC targeting moiety typically is a MAb or a MAb-like entity, such as a single-chain variable fragment (scFv) or bispecific antibody (bsAb). Herein, we generalize all those as MAbs for simplicity, except where specificity is needed.
Originally, the MAbs used clinically as targeting moieties in immunotoxins were of murine origin. However, second- and third-generation ADCs use chimeric, humanized, and fully human MAbs to reduce immunogenicity. A key MAb attribute is the ability to be internalized once arriving at a target cell. Internalization through RME is critical to therapeutic efficacy, enabling cytotoxins to act intracellularly.
Some recycling of MAbs and ADCs back into the extracellular environment occurs through the neonatal crystallizable fragment receptor (FcRn) pathway (9). For that and other reasons, ADC designs based on MAbs without Fc tails could have certain advantages. Another key goal for oncology indications is solid-tumor penetration. ADCs designed with traditional “large” MAb targeting moieties can have limited efficacy, a problem that might be mitigated by using scFv moieties or the Humabody technology of Crescendo Biologics. With a more compact structure than that of full-length MAbs, such constructs can penetrate tumors that are not well vascularized (13) but are still large enough to escape renal filtration.
Antibodies and antibody-derivatives play an important role in ADC constructs. Antibody size, rates of internalization, and antigen avidity potentially affect ADC functionality, so we briefly review those properties here. The smaller size of fragments could confer advantages over full-length antibodies. For example, a 150-kDa MAb has a different rate of diffusion into tissue and tumors than that of a 25-kDa scFv. The smaller fragment can bypass a patient’s lymphatic system when delivered extravascularly, which enables it to reach target tissue more efficiently. Using antibody fragments also could be more cost effective because of the option to use prokaryotic expression systems in manufacturing (15). Fab-containing antibody fragments have been successful in clinical applications, which makes them appealing to use in building ADCs.
Using smaller antibody fragments also brings disadvantages, however, such as faster clearance by a patient’s renal system and a lack of bivalency, which otherwise helps full-length MAbs target antigens. Because antibody fragments do not have Fc domains, lysosomal degradation and clearance necessitate more frequent or higher dosing of fragment-based ADCs (15). Single-chain variants (28 kDa) and single-domain antibodies (15 kDa) also could be considered for use as targeting moieties (15). Both types of antibody fragment can be multimerized, which is a positive attribute. Also, some manufacturing advantages come with using full-length antibodies, which can be purified using platform purification strategies based on protein A affinity chromatography capture. Without Fc domains, antibody fragments require other capture methods in downstream processing.
Therapeutic Targets: MAbs used as targeting moieties in ADCs are designed to bind specifically to upregulated cancer–cell-surface receptors with high avidity, thus delivering cytotoxins directly via cancer-cell antigens (or neoantigens) with minimal toxicity to normal tissues. Ideal therapeutic targets are those antigens that are uniquely expressed at relatively high levels on cancer cells and/or with high levels of receptor recycling. In addition, targets must be selected carefully to enable ADC internalization so that cytotoxic payloads will be delivered intracellularly. Postbinding antigen behavior also should be considered because shedding of antigens from cancer cell surfaces after ADC binding could increase toxicity. Bispecific antigen binding can help in this regard (13).
Linkers: The importance of linker design was underestimated in the early days of ADC therapies; researchers now understand that linkers are critical elements. Important features include a linker’s capabilities to
• maintain stability of an ADC construct while in circulation
• undergo modifications for therapeutic index optimization
• release the correct amount of cytotoxic payload within the endosome or lysosome of the target cell.
Linkers can be designed as either cleavable or noncleavable, the functional difference being that cleavable linkers facilitate cytotoxin release within both endosomes and lysosomes (Figure 1). Noncleavable linkers are proteolytically degraded in lysosomes, which leave cytotoxins covalently bound to linkers and their cognate amino acids or oligopeptides. ADCs with noncleavable linkers have an absolute requirement to be trafficked to lysosomes, where complete antibody digestion can occur to release the linker–toxin (LT) with an amino acid attached. The entire construct thus becomes the active metabolite (16). Noncleavable linkers are not as widely used in industry because of their limited ability to release their drug payloads efficiently, which potentially reduces therapeutic efficacy and increases toxicity (17).
Cleavable linkers fall into four categories:
• acid-labile (e.g., hydrazone) linkers, which are subject to low-pH acid cleavage in lysosomes
• disulfide linkages, which dissociate in the low redox potential of early endosomes
• β-glucuronide linkers, which are cleaved by lysosomal β-glucuronidase (18)
• peptide linkers, which are cleaved proteolytically by lysosomal proteases (Table 1).
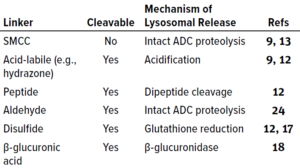
Table 1: Antibody–drug conjugate (ADC) linker chemistries; SMCC = succinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate.
The valine–citrulline dipeptide linker introduced by Seagen is the industry’s most frequently used peptide linker (9). Disulfide and hydrazone linkers are useful because they enable intracellular drug release while minimizing off-target toxicity. Disulfide and hydrazone linkers offer stability and selective intracellular cleavage, with the latter enabling tumor-specific release through pH sensitivity (9).
Most groups developing ADCs use such clinically proven linkers for targeted intracellular drug delivery in cancer therapy (17). Cleavable linkers also can be used for extracellular drug release, enabling delivery of drugs directly to target sites without the endocytosis requirement (17). However, that approach can limit drug penetration and diffusion through target tissues and cause off-target, dose-limiting toxicity (17).
The choice of coupling chemistry is important because it determines the site(s) of conjugation and number of toxins that will be conjugated to a targeting antibody — the drug/antibody ratio (DAR). The first ADCs used linker chemistries targeting lysine residues (Figure 2a), thus introducing multiple LTs per MAb because many potentially reactive lysines are present on the antibody surface. Moreover, because lysines are distributed throughout antibody domains in both variable and constant regions of the heavy and light chains, the potential of lysine-targeted chemistry to incorporate an LT into a variable region involved in antigen binding is relatively high compared with that of other conjugation chemistries.
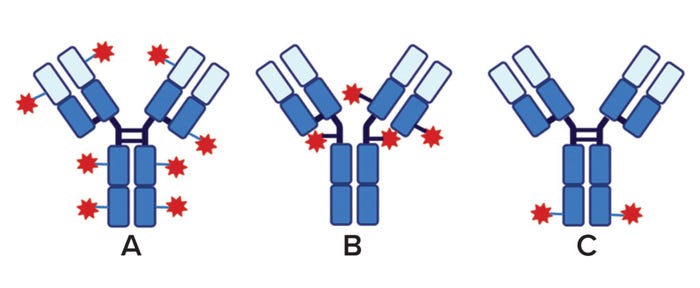
Figure 2: ADC types are defined by the number and site of attachment of linker–toxins (LTs) to the monoclonal antibodies (MAbs) for targeting. Here, MAb constant (dark blue) and variable regions (light blue) are shown, interchain disulfides are indicated by heavy black crosslinks, and the LT is represented by blue lines and red stars. (A) In a lysine-directed ADC with multiple, variable conjugation sites, as many as 40 lysine residues provide potential sites of conjugation in a MAb, and multiple LTs can attach to sites distributed over several domains. The drug/antibody ratio (DAR) is a broad range with an average generally >6. (B) In a cysteine-directed ADC, multiple but less-variable conjugation sites are derived from interchain cystines in partially reduced MAbs. The DAR is a narrow range (average DAR = 4). (C) Site-specific conjugation occurs at a single amino acid residue on each MAb heavy chain. This generally requires genetic engineering of the MAb to introduce a unique conjugation site. The average DAR is 2.
Because a MAb has fewer cysteine residues, for example, and because their distribution is more restricted and predictable, they are preferred over lysine as conjugation sites in current practice. Cysteine residues in MAbs primarily take the oxidized form of cystine, both as intrachain and interchain disulfides (Figure 2b) (19). The latter can be reduced selectively through reactivity with a number of reducing agents (e.g., TCEP), enabling selective conjugation of LTs to interchain disulfides.
An example of selective disulfide reduction is PolyTherics’ ThioBridge technology. In 2014, Badescu et al. described selective disulfide reduction using a sulfone reagent to rebridge disulfides (20). Such cysteine-directed technologies can produce consistently homogeneous drug products.
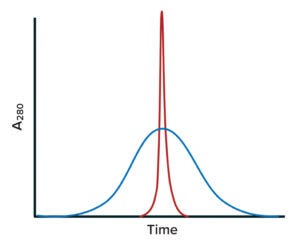
Figure 3: Idealized size-exclusion chromatography (SEC) results for stochastic and site-specific conjugates; ADC mixtures can be analyzed using SEC. The blue chromatogram trace is exemplifies a heterogeneous mixture of variants formed by insertion of payloads at multiple lysine residues, with drug/antibody ratios (DARs) greater than 6. Note the broad size distribution of conjugated forms. The red trace represents a nearly homogeneous preparation formed by site-specific insertion of a payload into an engineered location (average DAR = 2). Typically, such mixtures of conjugated forms are not fractionated during purification and thus compose the final product.
In recent years, companies have addressed the heterogeneity issue and improved both homogeneity (Figure 3) and manufacturing control of ADC constructs with site-specific conjugation of LTs (Figure 2c) (12, 21). Antibodies with engineered cysteines (e.g., Genentech/Roche’s Thiomab technology) have been used to create site-specific ADCs (22). Drake et al. describe an interesting example of site-specific linkage in Catalent’s SMARTag technology, through which a cysteine-containing pentapeptide sequence is engineered into a MAb structure (23). That sequence can be inserted at multiple locations in the amino-acid sequence. The host cell line used to express those modified MAbs is cotransfected with the gene for a formylglycine-generating enzyme that recognizes a pentapeptide sequence and enzymatically converts cysteines within it to formylglycine. That creates a unique aldehyde that serves as a specific conjugation site on the MAb (23).
Site-specific conjugation to individual endogenous residues can be achieved without such MAb engineering. For example, conserved glutamine residues in antibody Fc regions have been targeted for site-specific ADC conjugation, in which transglutaminase directs the site specificity (21). In another example, Fc domain lysine residues were targeted by Fujii and coworkers at Ajinomoto Bio-Pharma Services (25). In the company’s Ajicap method, one of a pair of lysine residues (Lys248 and Lys288) in the Fc region is modified chemically using a peptide linker designed to bind specifically to neighboring residues and thus position a reactive moiety — either a thiophenyl ester or an alkylthioester — so that it will couple uniquely to the target lysine. The end result is an Fc region conjugated at a single point of attachment on each chain at Lys288 (or Lys 248) for a fixed DAR = 2.
In 2021, Walsh et al. reviewed a number of methods for site-specific conjugation (26): cysteine engineering, disulfide rebridging, noncanonical amino-acid insertions, C- and N-terminal modifications, chemical and enzymatic amino-acid modification, and glycan modification. Trends in ADC design have shifted very strongly toward such approaches. In the past decade, the proportion of site-specific conjugates entering clinical trials increased from <10% in 2011 to 100% in 2020 (21).
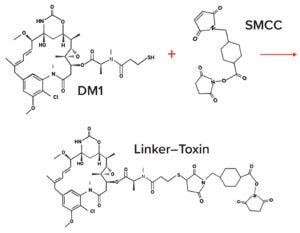
Figure 4A: Example linker–toxin (LT) coupling and ADC conjugation chemistries; linker and toxin generally are coupled together as a synthetic unit. Here, succinimidyl-4-(N-maleimidomethyl)cyclohexane-1- carboxylate (SMCC) is the linker, and mertansine (DM1) is the cytotoxin. Maleimide chemistry is used to link the free sulfhydryl group of DM1 covalently with the maleimide group on the SMCC linker to form a thiosuccinimide LT.
Cytotoxins: The cytotoxins used in ADCs have been used as solo chemotherapeutic agents, themselves. The selected drug should be toxic at a subnanomolar concentration because the efficiency of delivering it to target cells is low otherwise, usually <0.1% of administered dose per gram of tumor (12). To date, two general classes of cytotoxins have been used in ADC design: microtubule inhibitors and DNA-damaging agents. Examples of the former used in ADCs include auristatins (e.g., monomethyl auristatins E and F) and maytansinoids (9) (Figure 4). DNA-damaging agents include calicheamicins, duocarmycins, doxorubicin, and pyrrolobenzodiazepines (9, 24). A third class of cytotoxins, topoisomerase 1 inhibitors, is the most recent to be approved by FDA (27).
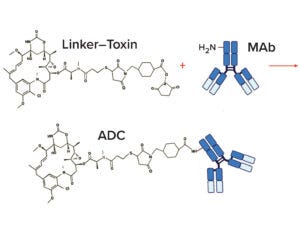
Figure 4B: Example linker–toxin (LT) coupling and ADC conjugation chemistries; a separately prepared monoclonal antibody (MAb) is conjugated to the LT. At neutral pH, the N-hydroxysuccinimide group on the LT reacts with a free amino group on the MAb to create an ADC. This example shows conjugation of a single LT for illustrative purposes.
To prevent dose-limiting toxicity from premature cytotoxin release, the LT must remain intact while an ADC is in circulation, but the linker also must allow for payload release once the drug is delivered to its site of action (11). Extracellular release in the region surrounding a tumor may offer some advantages (the “bystander effect”), but those come with some normal tissue toxicity that narrows the therapeutic window. Thus, considerations of LT stability are important for preventing premature ADC cleavage and cytotoxin release into circulation by maintaining integrity and desirable pharmacokinetic profiles of the conjugated molecule.
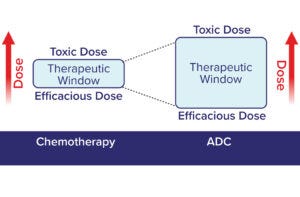
Figure 5: The therapeutic window for a cytotoxic chemotherapeutic agent is broadened by delivery as part of an ADC (12). Traditional chemotherapies require a higher efficacious dose and have a relatively narrow therapeutic window compared with ADCs.
Therapeutic Window: The difference between an efficacious dose and one that causes damage to normal tissues defines a drug’s therapeutic window. A drug candidate is considered to be promising if that difference is large. Traditional chemotherapies have characteristically narrow therapeutic windows because they are inherently cytotoxic and lack specificity for tumor cells (Figure 5). By contrast, incorporating those same chemotherapeutic agents as ADC payloads can deliver them to tumors selectively, broadening their therapeutic index. The resulting ADCs can be efficacious at lower doses and across broader dose ranges than those of the same cytotoxins administered alone as chemotherapeutics. That is not to say that the payloads cease to be highly potent compounds; indeed, dose-limiting toxicity remains a key challenge in the ADC field (28, 29).
Different conjugation chemistries alter the pharmacokinetics and therapeutic windows of ADCs. Conventional drug-conjugation strategies are stochastic and produce a heterogeneous mixture of ADC molecules with different DARs and variable pharmacokinetics, efficacy, and safety profiles. ADCs with high DARs can destabilize and aggregate, causing increased off-target toxicity and enhanced clearance from systemic circulation. Thus, using site-specific conjugations — e.g., methods to enable enzymatic conjugations and insertion of engineered cysteine residues or nonnatural amino acids in the antibody sequence — is increasingly popular among developers of ADC therapeutics.
Manufacturing Challenges
Because two intermediates are needed to produce an ADC — the LT and the MAb — manufacturing of these products presents some unique challenges. By themselves, most MAbs are amenable to standard platform technologies. However MAb biomanufacturing is just one stage of a multistep ADC process (Figure 6), which often involves different contract development and manufacturing organizations (CDMOs) and facilities. Their activities must be coordinated for timely generation of the final drug products for regulatory approval by health authorities.
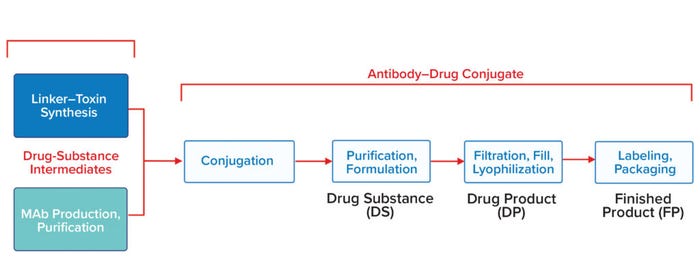
Figure 6: In this generalized process flow for ADCs, manufacturing starts with production of two drug-substance intermediates: a linker–toxin (LT) by organic synthesis or semisynthesis and a monoclonal antibody (MAb) by cellculture production and downstream processing (far left). The integrated LT is chemically conjugated with the purified MAb to form an ADC, which is purified and formulated to produce the drug substance (DS). Sterile filtration, filling into vials, and lyophilization yield the drug product (DP). Packaging and labeling of the DP yield a finished product (FP).
Antibody- and Linker-Related Challenges: ADC stability is based not only on the structure of a constituent MAb, but also importantly on its corresponding LT. If the latter is unstable, then an ADC can degrade in the vial and ultimately produce free cytotoxins that can reduce the product’s therapeutic window upon administration to patients. For optimized stability and product quality, ADC preparations often are lyophilized even in early stage process development.
Cytotoxin Challenges: Many early ADCs involved cytotoxins with relatively low potency (11). Given the generally low degree of drug delivery to target tissues, highly potent drugs should be used. Good examples include auristatins, maytansinoids, and calicheamicins. Development and scale-up of those toxins as payloads present significant challenges for process chemists. ADC payload molecules are cytotoxins derived from natural products and thus exhibit high molecular complexity, requiring long and complicated synthetic routes of production. The most common strategy is integrated synthesis of linker and toxin so that they are produced as a single LT intermediate.
Additionally, the toxicity of such molecules necessitates that the final steps in their production be conducted in high-containment facilities, which adds challenges to both development and manufacture. However, high potency significantly reduces the amount of LT required for commercial production (kilograms rather than tons) relative to that of a standard chemotherapeutic drug. Thus, synthetic methodologies and technologies that otherwise might not be considered by process chemists can be used for ADCs. Additionally, with extensive schemes needed to make LTs as single compounds, excellent opportunities exist to introduce new methods, to develop and optimize reactions, and to use other process chemistry expertise for improving the cost, quality, and speed of LT manufacturing (30).
Formulation Challenges: ADCs present unique formulation challenges because of their complex, multicomponent structures. Because each ADC drug substance is unique, platform formulations used for standard MAbs may not apply. That creates a need for individually optimized formulations (31). The target specificity of ADCs enables administration at lower concentrations than those required by standard MAb treatments, thus reducing aggregation risk. Conversely, the LT components increase hydrophobicity of ADCs, which can increase aggregation risk (31). The latter effect is exacerbated in ADCs with high DARs. Many marketed ADCs are lyophilized, which decreases hydrolysis of the linkers but presents other formulation challenges including stabilizer dilution upon reconstitution (31).
Formulation pH presents another difficulty for ADCs, particularly those that rely on endosomal acidification for cytotoxin release and for SMCC-linked ADCs that are sensitive to elevated pH (31). Surfactants typically are used to stabilize such products, but polysorbates can degrade upon storage and are challenging to characterize (31). Because ADCs often are administered by infusion, in-use stability (including compatibility with infusion-kit plastics and other components) becomes an important consideration. Taken together, all these aspects of ADCs become critical to establishing suitable formulations that allow for safe administration while maintaining product stability during storage and administration.
Manufacturing Complexity: ADCs are complex, multicomponent drug products combining two separate and distinct good manufacturing practice (GMP) intermediates — antibodies and LTs — each of which requires its own CMC technologies and capabilities. LT production involves complex chemistries. Chemical safety considerations also come into play when companies work with highly potent compounds such as cytotoxins. Those issues limit the number of manufacturers that can produce LT components.
The conjugation step required to attach an LT to a MAb presents yet another manufacturing hurdle. Unconjugated MAb and excess cytotoxins will be present in the conjugation-reaction mixture and must be removed to generate bulk drug product. Excess cytotoxin can be removed by methods such as tangential-flow filtration (TFF), but removing unconjugated MAb requires chromatographic separations. Because LT addition generally increases the hydrophobicity of an ADC, the purification method of choice often will be preparative hydrophobic-interaction chromatography (HIC). If the increase in hydrophobicity is proportional to the number of LT moieties added, then that method also can resolve ADC species with different average DARs to fractionate the conjugation mixture. If HIC is used, then a developer must decide which fractions will be combined to constitute the DP. Thus, unconjugated MAb (average DAR = 0) can be excluded from the ADC product pooling strategy.
MAb biomanufacturing involves cell-line and process development for the recombinant protein, then production scale-up and downstream processing of clinical material. These are all separate manufacturing operations: LT synthesis and conjugation to the resulting MAb (thus creating the drug substance), then filling of the formulated drug product, and finally labeling and clinical packaging of filled vials. The work often is performed by three or more different service providers, whose activities must be coordinated by a product sponsor.
The multiple components required to manufacture an ADC introduce logistical complexity beyond what is encountered for clinical MAb manufacturing. For an ADC, each intermediate requires specialized skills:
• A cell line and process must be designed for the MAb, and it must be produced as a purified intermediate (essentially through to clinical DS, although it is not designated in this way)
• The LT must be designed and synthesized, usually as a single chemical unit
• That will be supplied to a CDMO that performs the conjugation reaction and further purification (e.g., HIC and TFF) to produce the DS
• DS is formulated and filled as a DP
• Filled vials are labelled and packaged.
Thus, the ADC supply chain can involve as many as five different companies whose activities must be coordinated by the sponsor. A number of CDMOs can provide more than one of the required services. Sometimes a single service provider can accomplish clinical MAb production, LT synthesis, and conjugation to produce drug substance of the highly potent compound, then fill–finish of drug product. For evaluating CDMOs for multiple services, we advise that sponsors assess their capabilities across the required services to determine how well they compare with a strategy of separately contracting those services to specialized providers.
Analytical Challenges: Investigational new drug (IND)–enabling release/stability testing and characterization of therapeutic conjugates require specialized expertise and methods (including instrumentation and algorithms) to accommodate the unique molecular structures (20, 32). Understanding of both small and large molecules is needed to ensure consistent manufacturing, product and process quality, and ultimately drug safety and efficacy for patients. In our experience, the best overall strategy is to make parallel comparisons between the LT and ADC, and between the MAb and ADC, which will reveal critical quality attributes (CQAs) that are analytically consistent across molecular components and methods (33). Table 2 lists some key examples. The preference is for early release/stability test methods that can bridge easily to more hyphenated (specific) characterization methods.
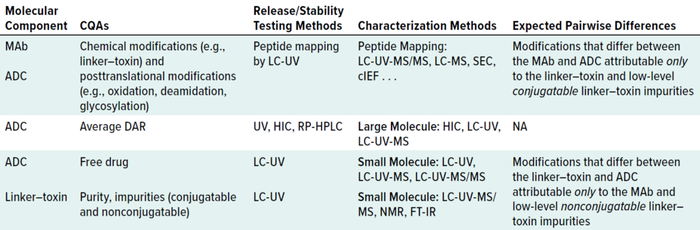
Table 2: Analytical methods for ADC development and characterization; CQA = critical quality attribute, MAb = monoclonal antibody, LC = liquid chromatography, UV = ultraviolet-light detection, MS = mass spectrometry, HIC = hydrophobic-interaction chromatography, RP = reversed phase, SEC = size-exclusion chromatography, NMR = nuclear magnetic resonance, DAR = drug/antibody ratio, FT-IR = Fourier-transform infrared, cIEF = capillary isoelectric focusing, NA = not applicable.
Many CDMOs can produce MAbs, but as noted above, very few have the end-to-end capability to manufacture antibodies, LTs, and final conjugated drug products. Analytical as well as logistical challenges arise when as many as five separate CDMOs are engaged to complete GMP manufacturing of an ADC. With the recent uptick in ADC development, more CDMOs with full ADC capabilities are likely to come online. A few “one-stop shops” already provide ADC development and manufacturing.
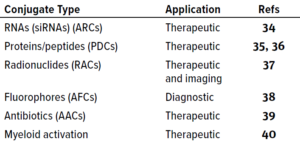
Table 3: Examples of antibody conjugates with payloads other than cytotoxins.
Payloads Other Than Cytotoxins: As summarized in Table 3, Panowski et al. noted in 2014 that MAbs can be used to deliver payloads other than cytotoxic drugs (12). Some carry silencing RNAs (34) or therapeutic peptides (35, 36). Radionuclide-chelator (37) and fluorophore (38) antibody conjugates are used in radio- and fluorescence-imaging, and antibody–antibiotic conjugates are being evaluated as antibacterial therapies (39). In our consulting work, we have been involved with several clinical programs based on such promising constructs.
Future Directions
The biopharmaceutical industry has made great progress in treating cancer over the past several decades. ADCs provide a widening range of tools that are proving to be useful in oncology. Since the first immunotoxins were made decades ago, advances in our understanding of key elements in ADC design include development of MAbs with increased specificity and affinity, linkers with improved stability, and toxins with intensified potency. Those have allowed the industry to create ADCs as commercial products that are altering clinical outcomes and improving patients’ lives. Undoubtedly, new applications for conjugated antibody products will be developed in the future. New technologies for receptor targeting, linker chemistries, and payload concepts will be discovered and applied to future ADC products. Improved technologies come alongside discoveries of new internalizing disease targets that are amenable to the use of ADCs. We expect the field to continue expanding so that ADCs can improve the lives of more patients in the years to come.
References
1 Ehrlich P. Experimental Researches on Specific Therapy: On Immunity with Special Reference to the Relationship between Distribution and Action of Antigens. The Collected Papers of Paul Ehrlich. Himmelweit F, Ed. Pergamon Press, Oxford, UK, 1960: 106–117.
2 Strebhardt K, Ullrich A. Paul Ehrlich’s Magic Bullet Concept: 100 Years of Progress. Nat. Rev. Cancer 8(6) 2008: 473–480; 20080512; https://doi.org/10.1038/nrc2394.
3 Ghetie V, et al. Large Scale Preparation of Immunotoxins Constructed with the Fab’ Fragment of IgG1 Murine Monoclonal Antibodies and Chemically Deglycosylated Ricin A Chain. J. Immunol. Meth. 112(2) 1988: 267–277; https://doi.org/10.1016/0022-1759(88)90367-5.
4 Chaudhary VK, et al. A Recombinant Immunotoxin Consisting of Two Antibody Variable Domains Fused to Pseudomonas Exotoxin. Nature 339(6223) 1989: 394-397; https://doi.org/10.1038/339394a0.
5 Bacha P, et al. Interleukin 2 Receptor-Targeted Cytotoxicity: Interleukin 2 Receptor-Mediated Action of a Diphtheria Toxin-Related Interleukin 2 Fusion Protein. J. Exp. Med. 167(2) 1988: 612–622; https://doi.org/10.1084/jem.167.2.612.
6 Vitetta ES, et al. Phase I Immunotoxin Trial in Patients with B-cell Lymphoma. Cancer Res. 51(15) 1991: 4052–4058.
7 Leshem Y, Pastan I. Exotoxin Immunotoxins and Anti-Tumor Immunity: From Observations at the Patient’s Bedside to Evaluation in Preclinical Models. Toxins (Basel) 11(1) 2019; 20190105; https://doi.org/10.3390/toxins11010020.
8 Prince HM, et al. Phase III Placebo-Controlled Trial of Denileukin Diftitox for Patients with Cutaneous T-Cell Lymphoma. J. Clin. Oncol. 28(11) 2010: 1870–1877; 20100308; https://doi.org/10.1200/JCO.2009.26.2386.
9 Khongorzul P, et al. Antibody–Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 18(1) 2020: 3–19; 20191028; https://doi.org/10.1158/1541-7786.
10 Tong JTW, et al. An Insight into FDA Approved Antibody–Drug Conjugates for Cancer Therapy. Molecules 26(19) 2021: 5847; https://doi.org/10.3390/molecules26195847.
11 Sievers E, Senter P. Antibody Drug Conjugates in Cancer Therapy. Ann. Rev. Med. 64, 2013: 15–29; https://doi.org/10.1146/annurev-med-050311-201823.
12 Panowski S, et al. Site-Specific Antibody Drug Conjugates for Cancer Therapy. MAbs 6(1) 2014: 34–45; https://doi.org/10.4161/MAbs.27022.
13 DePalma A. Special Report on Antibody–Drug Conjugates: Technical Challenges and Opportunities. BioProcess Int. eBook 14 November 2016: https://bioprocessintl.com/2016/special-report-antibody-drug-conjugates-technical-challenges-opportunities.
14 Jain N, et al. Current ADC Linker Chemistry. Pharm. Res. 32(11) 2015: 3526–3540; https://doi.org/10.1007/s11095-015-1657-7.
15 Goulet DR, Atkins WM. Considerations for the
Design of Antibody-Based Therapeutics. J. Pharm. Sci. 109(1) 2020: 74–103; https://doi.org/10.1016/j.xphs.2019.05.031.
16 Frigerio M, Camper N. Linker Design and Impact on ADC Properties. Chemical Linkers in Antibody Drug Conjugates. Van Delft FL, Lambert JM, Eds. Royal Society of Chemistry: Cambridge, UK, 2022; 71–121.
17 Bargh JD. Cleavable Linkers in Antibody–Drug Conjugates. Chem. Soc. Rev. 48(16) 2019: 4361–4374; https://doi.org/10.1039/c8cs00676h.
18 Jeffrey S, et al. Development and Properties of Glucuronide Linkers for Monoclonal Antibody-Drug Conjugates. Bioconjugate Chem. 17, 2006: 831–840; https://doi.org/10.1021/bc0600214.
19 Hermanson GT, van Delft FL. Antibody Conjugation Technologies. Chemical Linkers in Antibody Drug Conjugates. Van Delft FL, Lambert JM, Eds. Royal Society of Chemistry: Cambridge, UK, 2022: 32–65.
20 Badescu G, et al. Bridging Disulfides for Stable and Defined Antibody Drug Conjugates. Bioconjug. Chem. 25(6) 2014: 1124–1136; https://doi.org/10.1021/bc500148x.
21 Sadiki A, et al. Site-Specific Conjugation of Native Antibody. Antib. Ther. 3(4) 2020: 271–284; 20201218; https://doi.org/10.1093/abt/tbaa027.
22 Adhikari P, et al. Site-Specific Conjugation to Cys-Engineered THIOMAB™ Antibodies. Meth. Mol. Biol. 2078, 2020:51–69; https://doi.org/10.1007/978-1-4939-9929-3_4.
23 Drake PM, et al. Aldehyde Tag Coupled with HIPS Chemistry Enables the Production of ADCs Conjugated Site-Specifically to Different Antibody Regions with Distinct In Vivo Efficacy and PK Outcomes. Bioconjug. Chem. 25(7) 2014: 1331–1341; https://doi.org/10.1021/bc500189z.
24 Jackson PJM, et al. Use of Pyrrolobenzodiazepines and Related Covalent-Binding DNA-Interactive Molecules As ADC Payloads: Is Mechanism Related to Systemic Toxicity? Drug Discov. Today Technol. 30, 2018: 71–83; 20181206; https://doi.org/10.1016/j.ddtec.2018.10.004.
25 Fujii T, et al. AJICAP Second Generation: Improved Chemical Site-Specific Conjugation Technology for Antibody–Drug Conjugate Production. Bioconjug. Chem. 34(4) 2023: 728–738; https://doi.org/10.1021/acs.bioconjchem.3c00040.
26 Walsh SJ, et al. Site-Selective Modification Strategies in Antibody–Drug Conjugates. Chem. Soc. Rev. 50(2) 2021: 1305–1353; 20201208; https://doi.org/10.1039/d0cs00310g.
27 Conilh L, et al. Payload Diversification: A Key Step in the Development of Antibody–Drug Conjugates. J. Hematol. Oncol. 16(3) 2023: https://doi.org/10.1186/s13045-022-01397-y.
28 Hamblett KJ, et al. Effects of Drug Loading on the Antitumor Activity of a Monoclonal Antibody Drug Conjugate. Clin. Cancer Res. 10(20) 2004: 7063–7070; https://doi.org/10.1158/1078-0432.CCR-04-0789.
29 Nguyen TD, Bordeau BM, Balthasar JP. Mechanisms of ADC Toxicity and Strategies To Increase ADC Tolerability. Cancers (Basel) 15(3) 2023; https://doi.org/10.3390/cancers15030713.
30 Goundry WRF, Parker JS. Payloads for Antibody–Drug Conjugates. Org. Proc. Res. Dev. 26(8) 2022: 2121–2123; https://doi.org/10.1021/acs.oprd.2c00227.
31 Duerr C, Friess W. Antibody–Drug Conjugates: Stability and Formulation. Eur. J. Pharmaceut. Biopharmaceut. 139, 2019: 168–176; https://doi.org/10.1016/j.ejpb.2019.03.021.
32 Wakankar A, et al. Analytical Methods for Physicochemical Characterization of Antibody–Drug Conjugates. MAbs 3(2) 2011: 161–172; https://doi.org/10.4161/MAbs.3.2.14960.
33 Linz T, et al. Systematic LC/MS/MS Investigations for the IND-Enabling Extended Characterization of Antibody–Drug Conjugate Modifications. Antibodies (Basel) 7(4) 2018; https://doi.org/10.3390/antib7040040.
34 Klein D, et al. Centyrin Ligands for Extrahepatic Delivery of siRNA. Mol. Ther. 29(6) 2021: 2053–2066; https://doi.org/10.1016/j.ymthe.2021.02.015.
35 Gillies SD, et al. Expression of Genetically Engineered Immunoconjugates of Lymphotoxin and a Chimeric Anti-Ganglioside GD2 Antibody. Hybridoma 10(3) 1991: 347–356; https://doi.org/10.1089/hyb.1991.10.347.
36 Ronca R, et al. Delivering Cytokinesattumorsite: The Immunocytokine-Conjugated Anti-EDB-Fibronectin Antibody Case. Immunobiol. 214(9–10) 2009: 800–810; https://doi.org/10.1016/j.imbio.2009.06.005.
37 Bodei L, et al. Radiotheranostics in Oncology: Current Challenges and Emerging Opportunities. Nat. Rev. Clin. Oncol. 19(8) 2022: 534–550; https://doi.org/10.1038/s41571-022-00652-y.
38 Mao SY, Mullins JM. Conjugation of Fluorochromes to Antibodies. Methods Mol Biol. 588, 2010: 43–48; https://doi.org/10.1007/978-1-59745-324-0_6.
39 Mariathasan S, Tan MW. Antibody–Antibiotic Conjugates: A Novel Therapeutic Platform Against Bacterial Infections. Trends Mol. Med. 23(2) 2017: 135–149; https://doi.org/10.1016/j.molmed.2016.12.008.
40 Ackerman SE, et al. Immune-Stimulating Antibody Conjugates Elicit Robust Myeloid Activation and Durable Antitumor Immunity. Nat. Cancer. 2(1) 2021: 18–33; https://doi.org/10.1038/s43018-020-00136-x.
Peter Alexander is a senior consultant, Kelly Weitzel is a project manager, Angela Linderholm is a consultant, William E. Haskins and Sanjeevani Ghone are senior consultants, and corresponding author Steven M. Chamow is senior vice president — all in CMC at Alira Health, 1 Grant Street, Suite 400, Framingham, MA, 01702 USA; 1-417-459-1334; [email protected]; https://alirahealth.com.
You May Also Like



.jpg?width=700&auto=webp&quality=80&disable=upscale)

