- Sponsored Content
- Bioprocess Insider

Understand viral titer and qualityUnderstand viral titer and quality
The cell and gene therapy field has burgeoned in the last decade with drug candidate treatments for many different diseases.
June 13, 2022

Sponsored by Mirus Bio
The cell and gene therapy field has burgeoned in the last decade with drug candidate treatments for many different diseases. Determining the correct patient dosage has become more important than ever to extend the reach of the multi-million-dollar manufacturing runs required to generate these viral therapies.
Genome copies (GC), capsids, GC/capsid ratios, percent full, functional titering… What does it all mean? As of today, there is no single perfect method for capturing the qualities of viral therapies. Thus, manufacturers often use multiple approaches to verify the viruses they are producing are of high quality and functionality, appropriate for use in patients.
Methods to measure lentivirus and adeno-associated virus (AAV)
Akin to measuring the activity of traditional pharmaceutical drugs, determining viral titer is essential for understanding the infectious potential of viral therapies. Several established methods exist for reliably quantifying physical (viral proteins/genomes) or functional (infectivity) titer (Table 1).
Table 1. Lentivirus and AAV Titering Methods
Method | Measurement | Units | Notes |
|---|---|---|---|
Flow cytometry | Number of transduced cells | Transducing units (TU)/ml | Infectivity is measured by the number of transduced cells expressing viral genes. |
Number of viral particles | Virus particles/ml | Viral particles immobilized on beads or antibodies are counted. | |
qPCR/dPCR | Molecules of lentiviral RNA | Genome copies/ml | The quantity of viral genomes within harvested virus samples is measured. |
Molecules of AAV DNA | |||
Copies of integrated lentiviral DNA | Copies/cell | Infectivity is determined by measuring the quantity of viral genomes in transduced cells. | |
Copies of replicated AAV DNA | |||
ELISA | Viral proteins (e.g. capsid epitope) | Varies, typically pg/ml | Readout relies on antibody binding directly or indirectly to viral protein. |
Surface Plasmon Resonance | Viral protein binding | Virus particles/ml | Changes in the refractive index of a surface upon binding of virions are measured. |
Tunable Resistive Pulse Sensing | Number of viral particles | Virus particles/ml | Size and concentration of single particles are measured as they pass through a nanopore. |
Electron Microscopy | Number of viral particles | Virus particles/ml | Viral particles are visualized and counted. |
Choice of method matters
A common method for determining viral titer is using PCR to quantify genome copies. This method provides bare minimum gene expression data with relative ease. Though more time-consuming and costly to develop and perform, a functional assay may provide the most relevant information regarding a viral prep’s ability to transduce cells.
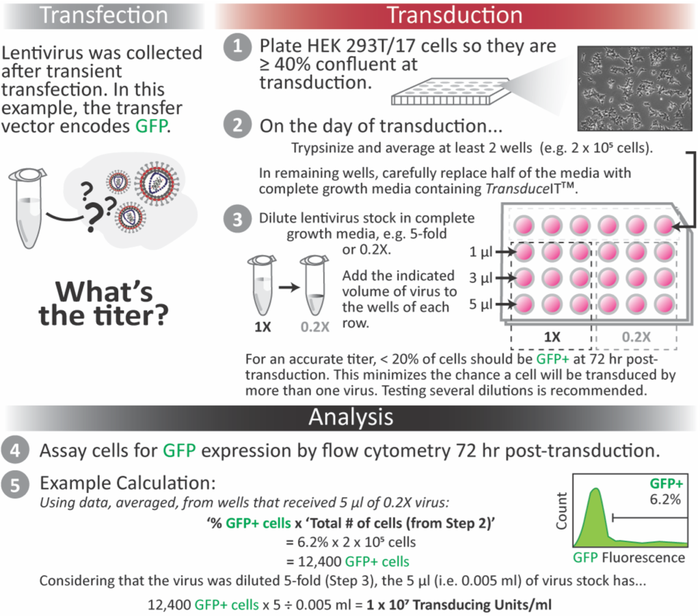
Infection assays should include the use of a permissible cell line, ideally as close as possible to the actual target of the produced AAV or lentivirus vector. Other features of assay design can vary depending on optimized conditions such as when to assay post-transduction (i.e. 48-72 hours).
Methods to measure viral quality
Quality and safety are critical factors when considering viral therapies for human use. Crucial components of the manufacturing process can influence the effectiveness of a therapy and must be monitored at the appropriate points during production. The implementation of quality control testing that detects process- and product-related impurities is paramount to the drug’s success.
While there are many rigorous tests viral therapies go through prior to use in a patient, Table 2 highlights a few common assays to ensure quality of the recombinant vector preparation. Viral vector manufacturers go to great lengths to ensure they are producing a maximum number of viral particles that contain the correct, intact genome (i.e. % full), without introducing impurities such as host cell DNA, protein or residuals from transfection.
Table 2. Testing to Assess Production Quality of Viral Therapies
Test | Method | Units | Specification/Notes |
|---|---|---|---|
Residual host cell DNA | qPCR/dPCR | Varies, typically ng/dose | < 10* |
Residual host cell protein (HCP) | ELISA | Percent HCP | Varies; dependent on clinical outcome. |
Percent full particles | qPCR/dPCR + Capsid ELISA | GC/capsid | Varies; high percent full is usually desired. |
Transmission electron microscopy (TEM) | Visual or automated detection of empty, partial or full capsids | ||
Analytical ultracentrifugation (AUC) | Peak absorbance at 260 nm | ||
Size exclusion chromatography with multi-angle light scattering (SEC-MALS) | GC/capsid | ||
Identity and residual reagent** | Varies | Varies | Varies |
* Wright JF, Zelenaia O. Vector characterization methods for quality control testing of recombinant adeno-associated viruses. Methods Mol Biol. 2011;737:247-78.
**In collaboration with Charles River Laboratories and Element Materials Technologies, Mirus Bio customers working with TransIT-VirusGEN® Transfection Reagent will be able to access Residual Reagent and Identity assays, respectively. Please contact [email protected] for more information.
Accuracy in titer and quality is important for downstream applications
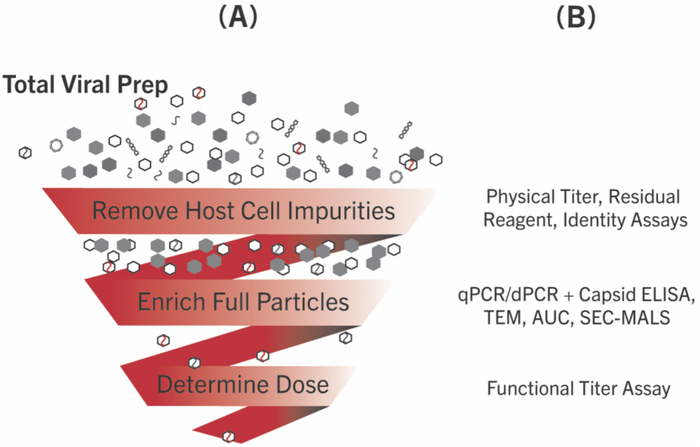
Figure 1. Assaying Titer and Quality in Therapeutic Vector Production Processes After transfection the (A) crude cellular lysate and/or media, i.e. Total Viral Prep, undergoes further processing and (B) testing at various points before use in therapies.
Total viral preps are full of impurities, including host cell DNA and protein, untransfected plasmid, unpackaged viral DNA or RNA and empty or partially filled capsids (Figure 1). Process development groups put forth great effort to remove these impurities to maximize doses. The assays described in this Tip from the Bench are performed prior to and/or congruently with each step of processing to provide information about the specific viral therapy produced.
Regardless of whether a manufactured viral therapy will be used in vivo or ex vivo, accurate titer measurements of high-quality material are critical. Some target cell types require a high multiplicity of infection to be transduced and treated and others may not. For particles administered directly to patients, the delicate balance of therapeutic effect versus potential increased risk of immunotoxicity from empty or partially filled capsids or neutralization by antiviral antibodies remains at the forefront of producing safe, effective viral therapies. For many manufacturers, a combinatorial approach incorporating multiple methods of quantification and quality assessment has become the gold standard in ensuring accurate viral titers of high quality and safety for use in people.
About the Author
You May Also Like






