March 8, 2021

Precigen says the electroporation device used to make its UltraCAR-T therapies is a game changer for cell therapy production.
The US biotech spoke about the technology – the UltraPorator – during its recent Q4 call, citing US FDA approval for its use in the production of investigational UltraCAR-T therapies as a highlight of 2020.
According to CEO Helen Sabzevari, “UltraPorator is a potential game-changer for manufacturing of personalized UltraCAR-T therapies as it significantly reduces processing time and contamination risk.
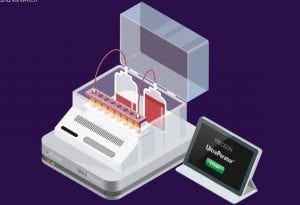
“While Precigen is utilizing it for internal programs currently, the UltraPorator system was designed to enable rapid manufacturing for a range of gene and cell therapies and has potential applications beyond UltraCAR-T manufacturing,” she told BioProcess Insider.
Commercial scale
Sabzevari explained that commercially available electroporation devices have limitations in system throughput and or gene transfer efficiency.
“This results in a labor-intensive process and a manual handling of samples that increase contamination risk and pose major challenges for scale-up and eventual commercialization for personalized cell therapies.”
UltraPorator is designed to address these limitations. According to Sabzevari It is capable of handling the electroporation of a large number of cells in a matter of minutes, as compared to multiple hours of processing currently required.
She added that, “the semi-closed system minimizes handling requirements, significantly reduces contamination risk, and streamlines the overnight manufacturing process. We believe that these allow us to bring the drug manufacturing process as close as possible to patients in a commercially viable and expedient way.”
Portfolio
Precigen is currently utilizing the UltraPorator system to manufacturing range of its UltraCAR-T therapeutic candidates.
The firm’s UltraCAR-T platform relies on reprograming cancer patient’s own T-cells to kill tumours. In November, Precigen dosed the first patients with UltraCAR-T cells manufactured using the UltraPorator system in the ongoing PRGN-3005 and PRGN-3006 Phase I clinical trials.
Sabzevari told us Precigen’s approach “is designed to overcome limitations of current generation of CAR-T therapies by utilizing an advanced overnight non-viral gene delivery manufacturing process at a medical centre’s cGMP facility.”
She added that, “Current CAR-T cell therapies are limited due to, inter alia, the prolonged interval between apheresis to product infusion and an exhausted phenotype of T cells resulting from lengthy ex vivo expansion.”
About the Author
You May Also Like




