Working Toward an AAV Platform: Challenges, Threats, and OpportunitiesWorking Toward an AAV Platform: Challenges, Threats, and Opportunities
Sponsored by Polyplus
Recent successes in gene therapies are driving continued expansion of the pipeline. The number of clinical trials using adenoassociated virus (AAV) and lentivirus (LV) vectors is growing each year. Currently underway are 669 AAV and 266 LV trials in the United States, 1,250 AAV and 94 LV trials in Europe, and 223 AAV and 140 LV trials in the Asia–Pacific region (1). And as target indications broaden, many candidates treat conditions that affect much larger patient populations than before, calling for systemic delivery with larger doses. These advances are increasing the demand for AAV and LV vector manufacturing.
One major challenge is that current viral-vector manufacturing processes suffer from poor yields — typically 30–40%. Fortunately, opportunities exist to improve the performance of both upstream and downstream operations. A great deal of attention has focused on improving purification strategies, but more investigation can and should be put into upstream production. Improvements in such contexts will influence the number of purification operations required downstream.
Manufacturing capacity can be increased. Companies can improve it by expanding existing sites or adding greenfield facilities; scaling existing processes; or intensifying processes. Process intensification improves efficiency and productivity processes to achieve higher-purity product in less time with a smaller manufacturing footprint.
Leveraging Lessons from Design of Experiments (DoE)
Platform approaches to monoclonal antibody (MAb) manufacturing are known to increase efficiency and productivity. The challenge for developing an AAV-vector manufacturing platform is to select among many different AAV serotypes for delivering gene therapies to different organs. Optimum plasmid transfection to produce serotypes and AAV vectors for therapeutic applications can require introduction of different cell lines, plasmid-construct designs, media, transfection process conditions, harvest methods, analytics, and so on.
Process parameters typically explored include the amount of plasmid DNA used, its ratio to transfection reagents, and the complexation volume and time. Those parameters should be evaluated for each process because slight changes in their values can result in measurable differences in productivity, titer, infectivity, and other attributes.
The best approach to optimizing transfection processes for a given AAV serotype and gene of interest is to use a design-of-experiments (DoE) strategy that enables simultaneous evaluation of multiple variables. DoE studies enable visualization of the interactions between factors, which is not possible with conventional one-factor-at-a-time (OFAT) studies.
Ensuring Process Optimization and Scalability
To identify the optimum transfection reagent for large-scale production of AAV vectors using suspension cell culture, Polyplus completed high-throughput screening of a large chemical library. The work focused on evaluating chemistries with the greatest potential and performance. The FectoVIR-AAV transfection reagent — launched in 2020 and expanded to good manufacturing practice (GMP) grade in 2021 — was selected for producing more doses per batch for a lower cost per dose. It provides higher titers than other candidates can, with simplified protocols, lowered complexation volumes, and improved transfection-complex stability. The reagent is compatible with different media, cell lines, and AAV serotypes and comes in both research and GMP grades. When compared with the original gold standard (the PEIpro transfection reagent) in validation studies, the FectoVIR-AAV reagent at least doubled titers, yielding a higher percentage of full capsids (Figure 1).
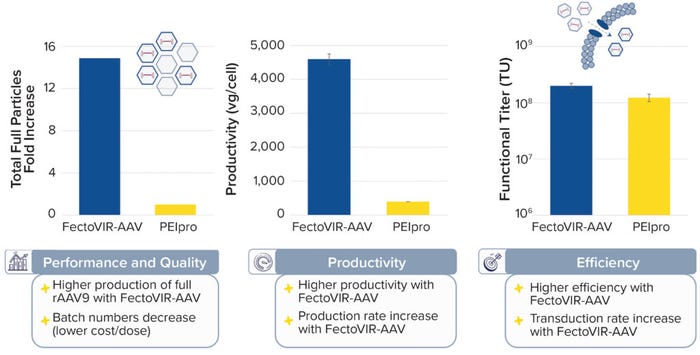
Figure 1: The FectoVIR-AAV reagent increases rAAV9 titers by at least twofold with a higher percentage of full capsids.
Upstream AAV PlatformDevelopment at ABL Europe
Contract development and manufacturing organization (CDMO) ABL Europe is working on a high-performing transient-transfection platform process using HEK293 cells that have been adapted for large-scale suspension cell culture (200 L and 500 L). The platform design is flexible to allow for modifications that maximize serotype production across differing scales of early to later-stage projects. This intellectual-property (IP)–free platform is meant for sharing scientific knowledge for the greater good.
To optimize the transient transfection process, ABL Europe evaluated the performance of both PEIpro and FectoVIR-AAV reagents with a Polyplus team. They implemented a DoE study using small-scale (125-mL) shake flasks to identify the critical process parameters (CPPs). After further DoE runs in small-scale bioreactors (the Ambr 250 modular system from Sartorius with up to eight vessels), the process was scaled to 2-L bioreactors.
Thawing and cell expansion, cell-line selection, cell growth, media selection, and passage numbers were optimized. For the transfection step, the team evaluated ratios of different plasmids, DNA concentration, cell-seeding density, transfection reagent, and transfection reagent:total DNA along with feed strategy, lysis conditions, and time of harvest. For the small-scale DoE studies, a two-plasmid system was used to produce AAV2, with lysis and clarification performed 72 hours posttransfection. Performance was evaluated based on the vector-genome quantity obtained as determined by quantitative polymerase chain reaction (qPCR).
With the FectoVIR-AAV product, the best titers were obtained at a DNA concentration of 0.5 µg/106 cells at time of transfection. In addition, the higher the plasmid ratio and cell-seeding density, the higher the titer (Figure 2). But the FectoVIR-AAV:DNA ratio did not affect titer. Processes using the optimum parameters were repeated in 125-mL shake flasks and run in Ambr 250 minibioreactors, giving highly reproducible results.
After adapting cells, the team used three different media to generate cell banks and ran processes using both reagents. With FectoVIR-AAV, there was no difference in the genomic titer, but the particle titer (determined using an enzyme-linked immunosorbent assay, ELISA) was reduced with two media. PEIpro use increased genomic titers with two media. It should be noted that although ELISA and qPCR are not optimal methods for measuring full/empty capsid ratios, they remain the methods of choice for upstream samples and are useful for comparing samples.
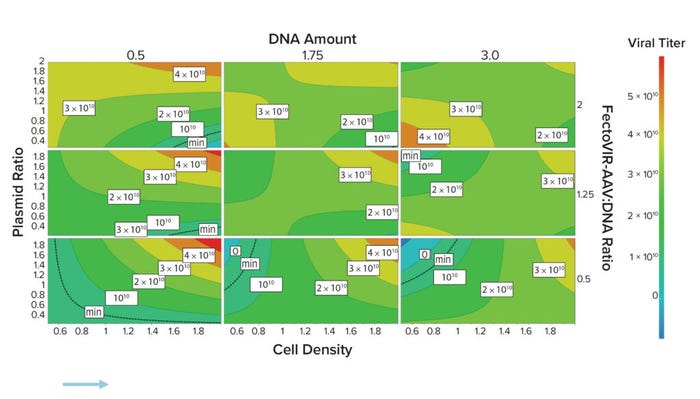
Figure 2 (top and bottom): A small-scale design of experiments (DOE) study (125-mL shake flask) showed that with the FectoVIR-AAV reagent, the highest AAV2 titers using a two-plasmid system were obtained at a DNA concentration of 0.5 μg/106 cells at time of transfection. Further DoE runs were then performed in small-scale bioreactors (Ambr 250, Sartorius), and the process was then scaled to 2-L bioreactors. Performance was evaluated based on the vector-genome quantity obtained, as determined by quantitative polymerase chain reaction.
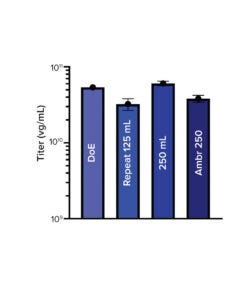 The process was repeated with three independent runs in a 2-L bioreactor. All provided expected titers in highly reproducible results (data not shown). The transfection process was repeated using the best parameters identified with the reagents to produce different AAV serotypes using an Ambr 250 modular small-scale bioreactor. As expected, the FectoVIR-AAV reagent gave higher AAV2 titers, and similar viral titers were observed for AAV8 with both transfection reagents. But higher genomic titers came for AAV5, AAV6, and AAV9 produced using FectoVIR-AAV reagent (Figure 3).
The process was repeated with three independent runs in a 2-L bioreactor. All provided expected titers in highly reproducible results (data not shown). The transfection process was repeated using the best parameters identified with the reagents to produce different AAV serotypes using an Ambr 250 modular small-scale bioreactor. As expected, the FectoVIR-AAV reagent gave higher AAV2 titers, and similar viral titers were observed for AAV8 with both transfection reagents. But higher genomic titers came for AAV5, AAV6, and AAV9 produced using FectoVIR-AAV reagent (Figure 3).
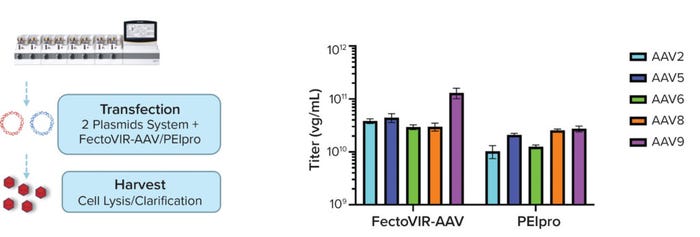
Figure 3: Performance using the best transfection parameters for FectoVIR-AAV and PEIpro reagents were assessed for production of AAV serotypes AAV2, AAV5, AAV6, AAV8, and AAV9.
In a new DoE using FectoVIR-AAV reagent for AAV8 production, the team identified different optimum transfection parameters than those identified for AAV2 and increased viral titers over fivefold (data not shown). That demonstrates the importance of optimizing each AAV production system.
A Powerful, Comprehensive Strategy
Increasing success with gene therapies and the movement toward such treatments for prevalent diseases is dramatically increasing the demand for viral vectors over what can be met with existing capacity. Although expansion can help meet some of this need, it is equally important to improve the efficiency and productivity of current manufacturing processes. The industry needs to produce more doses in intensified processes with smaller equipment.
A comprehensive DoE strategy with a platform approach can be powerful. From collaborations like this one between ABL Europe and Polyplus, the payout of productivity, safety, and efficacy in viral vector manufacturing is as clear as the benefit of using high-quality raw materials. As processes become further optimized, the industry will generate more functional virus in a smaller footprint with less reagent and media.
Open communication and clear documentation will help streamline the phases of DoE studies, from development of transfection processes through deciding, specifying, designing, collecting, analyzing, fitting, and predicting optimized conditions. It is important to understand how the CPPs for different transfection reagents will scale.
Finally, testing and assessing new technologies for AAV manufacturing will help to improve yields and purities further through more streamlined and robust processes. If the biopharmaceutical industry can find ways to maintain or increase quality with lowered costs of goods (and ultimately, costs per dose), then gene therapies have a greater chance of improving the lives of more patients.
Reference
1 Regenerative Medicine in 2021: A Year of Firsts and Records. ARM H1-2021 and Global Data; https://alliancerm.org/sector-report/h1-2021-report.
Mégane Denu is a bioproduction engineer, Polyplus (part of Sartorius); Quentin Bazot, PhD, is a viral vector manufacturing and process development specialist, ABL Europe; and corresponding author Alengo Nyamay’antu, PhD, is scientific communication team leader, Polyplus (part of Sartorius); [email protected].
You May Also Like



.jpg?width=700&auto=webp&quality=80&disable=upscale)

