Leishmaniasis — a Forgotten EpidemicLeishmaniasis — a Forgotten Epidemic
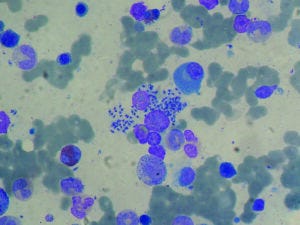
Bone marrow aspirate from a liver-transplant recipient showing infection with visceral leishmaniasis
(PAULO HENRIQUE ORLANDI MOURAO, HTTPS://WIKIPEDIA.ORG)
The World Health Organization (WHO) considers leishmaniasis to be one of the world’s most neglected tropical diseases (NTDs). As of January 2023, more than one billion people are at risk of infection with leishmaniasis because they live in endemic regions. NTDs disproportionately affect the world’s most underresourced and malnourished populations, contributing to a vicious cycle of poverty and disease. Yearly, between 498,000 and 862,000 new cases are diagnosed, resulting in over 18,700 deaths and up to 1.6 million disability-adjusted life years (DALYs) lost (1).
Leishmaniasis is a vector-borne disease caused by protozoan parasites of the genus Leishmania, which are transmitted through the bites of infected female sandflies and which manifest in three ways (2). Also known by the Hindi term kala-azar, meaning “black sickness,” visceral leishmaniasis (VL) affects spleen, liver, bone marrow, and other internal tissues. It is the most serious form of the disease. Cutaneous leishmaniasis (CL), the most common presentation of the disease, causes skin sores. Mucocutaneous leishmaniasis (ML) affects the nose, mouth, and throat. CL and ML are chronic and highly stigmatized conditions, yet they are not life threatening. Left untreated, VL is fatal in over 95% of cases. More than 90% of VL cases occur in endemic areas such as Brazil, Ethiopia, India, Kenya, Somalia, South Sudan, and Sudan (3). In addition, up to 50% of patients previously treated for VL develop post-kala-azar dermal leishmaniasis (PKDL), a chronic skin disease. PKDL patients are believed to be sources of Leishmania infections that lead to outbreaks and epidemics of VL, potentially sustaining the cycle of transmission even when small numbers of VL cases are reported.
Early diagnosis and treatment of active cases are the mainstay of leishmaniasis control. Still, available treatments can be costly, cause severe side effects, and contribute to the development of drug-resistant parasites (4). So far, preventative measures are restricted to vector control with insecticide-treated bed nets and indoor residual spray. No vaccine for human leishmaniasis is available yet. However, recovery from previous leishmaniasis infection leads to life-long immunity, making the development of a vaccine a valuable and attainable goal (5).
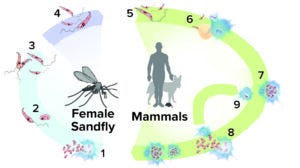
Figure 1: Life cycle of Leishmania parasites; first, parasites are transferred during sandfly feeding (1). Transferred amastigotes transform into procyclic promastigotes (2), which then change by simple division into metacyclic promastigotes (3). Those migrate to the host sandfly’s pharyngeal valve (4) and are transferred to a mammalian host during feeding (5). Metacyclic promastigotes enter into or are phagocytized by macrophages and granulocytes (6), where they mature into amastigotes and divide (7). After leaving their cellular hosts (8), the amastigotes infect new cells (9). (ADAPTED FROM MARIANA RUIZ VILLARREAL, HTTPS://WIKIPEDIA.ORG)
Vaccines: A Difficult Imperative
The quest for a VL vaccine has been hampered by the parasite’s significant antigenic diversity and complex life cycle, which must occur in at least two hosts (Figure 1), and by a lack of confidence in animal models as predictors of human vaccine response. Another significant consideration stems from the complexity of the immune reactions needed for protection. Although robust immune responses can accelerate recovery, some reactions can aggravate the disease. The type of response elicited differs by stage of disease progression, species of the infectious agent, and host immunity status. Such pathophysiological and immunological mechanisms must be well understood to develop a safe and effective vaccine.
Manufacturing technology represents another integral consideration for leishmaniasis vaccine programs. Such products will be administered predominately in low- and middle-income countries (LMICs). To ensure adequate market uptake, a leishmaniasis vaccine must be easy and cost-effective to manufacture, ideally using production processes that can be transferred to local vaccine manufacturers in LMICs. Thus, leishmaniasis vaccines might be well suited for incorporation into programs that the WHO, European Union, national governments, and even some pharmaceutical companies have established in the aftermath of COVID-19 for developing local vaccine-production capabilities in LMICs.
A Long Road to New Leishmaniasis Vaccines
Early efforts to develop preventive leishmaniasis vaccines were based on live–attenuated or inactivated Leishmania parasites (whole or fractions). More-recent attempts have used recombinant Leishmania proteins or protein fractions and chimeric peptides (based on in silico and in vitro analyses) (6). Numerous antigens have been applied in experimental vaccines. However, few candidates have been assessed in clinical trials, and none have exhibited adequate efficacy. Successful candidates will need to induce long-lasting and sufficiently powerful immune responses while achieving a delicate balance between T helper (Th) 1 and 2 cell immune responses. Doing so represents a major difficulty in developing a leishmaniasis vaccine.
Novel vaccine strategies are being applied to surmount such obstacles. One recent candidate is ChAd63-KH, which was developed by the University of York (UK). ChAd63-KH is based on a well-characterized simian adenovirus vector (ChAd63) expressing two Leishmania antigens, kinetoplastid membrane protein 11 (KMP-11) and hydrophilic acylated surface protein B (HASPB). The technology used in ChAd63-KH is similar to that of the Vaxzevria/Covishield coronavirus vaccine developed by Oxford University and AstraZeneca, which have transferred manufacturing successfully to the Serum Institute of India.
Preparation is underway to conduct a randomized placebo controlled phase 1b–2 clinical trial in Sudan, to be coordinated by the European Vaccine Initiative (EVI), a product-development facilitator with a proven track record in leading innovative solutions for vaccine research and development (R&D), capacity strengthening, and advocacy. The trial will determine whether vaccination of healthy adolescents with ChAd63-KH protects against VL/PKDL.
The project is intended to have implications beyond leishmaniasis, providing needed support for research into other NTDs through strengthening immunology research capacity among East African partners and members of the Leishmaniasis East Africa Platform.
As with other NTDs, leishmaniasis faces major difficulties in mobilizing adequate funding from national and international agencies to support vaccine development. Continued research and funding therefore remain critical factors to preventing a disease with significant global importance and social impact.
References
1 Abbafati C, et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 396(10258) 2020: 1204–1222; https://doi. org/10.1016/S0140-6736(20)30925-9.
2 Dasauni K, Bisht D, Nailwal TK. Novel Nanotechnology-Based Approaches in the Treatment of Leishmaniasis. Pathogenesis, Treatment, and Prevention of Leishmaniasis. Samant M, Pandey SC, Eds. Academic Press: Cambridge, MA, 2021, 125–140; https://doi.org/10.1016/B978-0-12-822800-5.00005-6.
3 Leishmaniasis Key Facts. World Health Organization: Geneva, Switzerland, 2022; https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
4 Alves F, et al. Recent Development of Visceral Leishmaniasis Treatments: Successes, Pitfalls, and Perspectives. Clin. Microbiol. Rev. 31(4) 2018: e00048-18; https://doi.org/10.1128/cmr.00048-18.
5 Das A, Nahid A. Vaccine Development Against Leishmania donovani. Front. Immunol. 3, 2012: 99; https://doi.org/10.3389/fimmu.2012.00099.
6 Kammona O, Tsanaktsidou E. Nanotechnology-Aided Diagnosis, Treatment, and Prevention of Leishmaniasis. Int. J. Pharm. 605, 2021: 120761; https://doi.org/10.1016/j.ijpharm.2021.120761.
Acknowledgments
Research described herein is part of the EDCTP2 Programme supported by the European Union (RIA2016V-1640; PREV_PKDL; https://prevpkdl.eu). The views and opinions of authors expressed herein do not necessarily state or reflect those of EDCTP.
Flavia D’Alessio is senior project manager, Sophie Houard is director of vaccine development, Stefan Jungbluth is head of business development, Romina Di Marzo is communications and advocacy manager, Catarina Luís is senior project and communications manager, and Ole Olesen is executive director of the European Vaccine Initiative, Universitätsklinikum Heidelberg, Voßstraße 2, Geb. 4040, 69115 Heidelberg, Germany; [email protected].
You May Also Like



.jpg?width=700&auto=webp&quality=80&disable=upscale)

