Detecting the Broad Spectrum of Pyrogens with the Human Whole-Blood Monocyte Activation TestDetecting the Broad Spectrum of Pyrogens with the Human Whole-Blood Monocyte Activation Test
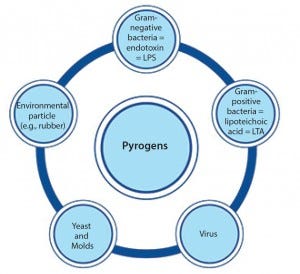
Figure 1: Pyrogens constitute a diversive heterogenous group of contaminants comprising microbial and nonmicrobial substances.
In the early 20th century, some patients injected with the drug Salvarsan experienced febrile reactions due to contamination of the drug’s distilled water. That incident (involving the first effective treatment for syphilis) prompted not only the widespread use of injectable drugs, but also the need for pyrogen control. Pyrogens constitute a heterogeneous group of microbial and nonmicrobial substances that include those derived from Gram-negative and Gram-positive bacteria such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA), respectively, as well as particles from yeasts and viruses (Figure 1). When present as contaminants in pharmaceutical products, pyrogens can activate the human immune system and induce life-threatening fever reactions.
The response of the human immune system to pyrogens is extremely fine-tuned, which explains the unusually consistent reaction among individuals. Of the hundreds of donors tested, no major deviations have been observed in the threshold concentrations of LPS that cause reactions and cytokine release (1). If the response to a pyrogen is not rigorous enough, infection will occur; and if the response is too much, inflammation will occur.
Monocytes contain cell-surface receptors for different pyrogenic substances and therefore regulate the induction of fever. For example, the endotoxin LPS (the most widely recognized and best understood pyrogen) elicits a particularly strong response from monocytes. In this case, the fever reaction starts when LPS binds to toll-like receptor 4 (TLR4) on a monocyte cell surface, setting off a cascade of intracellular reactions that ultimately result in the release of proinflammatory cytokines. Many additional receptors are present on a monocyte cell surface — including TLR2, TLR6, TLR7, TLR8, and TLR 9 — which trigger pyrogenic reactions upon binding of specific ligands. So it is essential that pharmaceuticals, medical devices, biotherapeutics, cell therapeutics, and cosmetics are tested for the full range of pyrogen contaminants to ensure human safety.
Three types of assays are available for pyrogen testing: the rabbit pyrogen test (RPTs), bacterial endotoxin tests (BETs), and monocyte activation tests (MATs). RPT and BETs are both animal-based tests. However, the MATs is a human-cell–based assay that most closely reflects the human immune response.
Pyrogen Detection Methods
Pyrogen detection Methods Rabbit Pyrogen Test: RPT was first developed in 1912 and ultimately accepted into a number of pharmacopoeias (e.g., European Pharmacopoeia 2.6.8, US Pharmacopeia <151>) in the 1940s. In brief, a sample is injected into rabbits and their change in body temperature is determined by means of rectal measurements.

Table 1: Comparison of approaches for monocyte activation tests
RPT can detect both endotoxin and nonendotoxin pyrogens (NEPs). Although rabbit blood is highly responsive to LPS, it is less responsive to Gram-positive pyrogens than human blood. That difference in rabbit responsiveness between Gram-negative and Gram-positive bacteria — which does not translate to humans — has misled pyrogen research for the past century to focus almost entirely on endotoxins (LPS) (1). Today, more than 60,000 scientific articles have been published on LPS, but only a few thousand on its Gram-positive counterparts.

Table 2: Comparison of rabbit pyrogen tests and monocyte activation tests for pyrogen contamination of human serum albumin (8); 29 different batches of albumin were tested pure and spiked with 5 or 10 IU/mL.
RPT provides false-negative responses to NEPs that are known to induce pyrogenic reactions in humans (2, 3). For example, in one study of 171 rabbits, researchers identified significant differences in the response rate of the animals to LPS, and they calculated the test to have only 58% specificity and 88% sensitivity. In a separate analysis, RPT was unable to accurately detect pyrogens spiked directly into human serum albumin, which leads to false-negative results (Table 2). In the same analysis, human MATs accurately detected pyrogens in all product batches (4).
Additional limitations to using rabbits for pyrogen testing include the fact that the assay is not quantitative. Also, the assay lacks a positive control and requires large numbers of animals to identify rare pyrogen-containing samples. And stress on the rabbits can influence results. RPT cannot be used to test many types of pharmaceutical products (e.g., chemotherapeutics and immunosuppressive agents), and they cannot be used to test human cellular preparations such as blood components and stem cells (5).
Bacterial Endotoxin Tests: BETs — also called Limulus amebocyte lysate (LAL) tests — refer to a number of tests that detect endotoxins from Gram-negative bacteria based on the clotting reaction of hemolymph in horseshoe crabs. This test can detect the most common and most potent pyrogen known to humans with high sensitivity. However, the biggest limitation of BETs/LAL tests is that they can detect only LPS.
BETs/LAL tests have been accepted into a number of pharmacopoeias (e.g., European Pharmacopoeia 2.6.14; US Pharmacopeia <85>) and expanded into broad use in the 1970s and 1980s. Over the past three decades, >90% of rabbit pyrogen testing for parenteral products has been replaced by BETs/LAL tests (1). However, in Europe >170,000 rabbits — and an estimated 400,000 worldwide — are used for RPT testing each year.
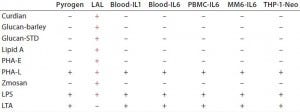
Table 3: Comparative analysis of pyrogen testing of nonendotoxin stimuli (7)
Nonendotoxin pyrogen contaminants that escape detection by this test may cause severe clinical problems (2). Table 3 shows the result of one comparative analysis conducted among RPT, BETs/LAL tests, and variants of MATs for nonendotoxin pyrogen detection. BETs/LAL tests were associated with false positives for b-glucan structures and false negatives for LTA.
An endotoxin masking effect also presents a barrier to accurate test results at low LPS concentrations. A large number of products may have transitioned from rabbit testing to BETs/LAL tests because of flawed product-specific validations, including insufficient time for endotoxin masking. Validation requires demonstrating the lack of interference of an endotoxin spike. However, the sample typically is spiked just before testing, which does not allow enough time for that effect to take place.
Another limitation of BETs/LAL tests is that these assays cannot be used to test solid materials (such as the surfaces of medical devices) or many types of liquid samples (such as dialysis fluids, liposomes, nanoparticles, and cell therapies). The assays also are not applicable to testing many vaccines because the common adjuvant, aluminum hydroxide, interferes with testing. Furthermore, BETs do not reflect the inflammatory potency of a specimen in humans (6).
Two national control authorities — the Norwegian Institute of Public Health (NIPH) and the UK National Institute for Biological Standards and Controls (NIBSC) — carried out BETs/LAL test studies in parallel with validation of MATs. The NIPH test results demonstrated 67% sensitivity and 100% specificity for BETs/LAL tests, and the NIBSC test results demonstrated 83% sensitivity and 33% specificity for the same test.
Monocyte Activation Tests: Because of the complexity of the human fever response, an assay based on fresh or cryopreserved human blood was developed and validated as a replacement for RPT and BETs (7, 8). Whole-blood MAT most closely reflects the human immune response, and it can detect both endotoxin and nonendotoxin pyrogens. The test is quantitative and can be applied to products that cannot be tested by RPT and BETs (e.g., medical devices). In brief, the test involves incubating whole blood with a sample (without the need for washing procedures for the cryopreserved blood) before researchers perform cytokine analysis. MAT works with either fresh or cryopreserved human blood. Such testing was accepted into the European Pharmacopoeia in 2010 (9).
Cell culture is not required for MATs because the cells are kept and maintained in their natural environment (plasma). Because cell suspensions are used, blood as a reagent can be brought into contact with any material, including medical devices or filters loaded with specimens from air samples. Many attempts have been made to replace the primary monocytes with cell lines to eliminate the need for a blood donor; however, cell lines demonstrate high variability, require cell culture, and have been shown to drift and shift in their responsiveness to pyrogens. Table 1 compares the sources of monocytes for use in MATs.
Extensive data generated over the past 20 years support the use of fresh whole blood assays for pyrogen testing. Those data include the analysis results from 90 different LPSs (endotoxins of Gram-negative bacteria), LTAs from 40 species of bacteria, five exotoxins, and 50 types of fungal spores. To date, the assay has been adapted for about 80 different products, including drugs, cell therapies, and medical devices. Supportive data also exist for the use of cryopreserved whole blood in testing different nonendotoxin pyrogens and routine product testing.

Table 4: Predictive capacity of using cryopreserved whole blood in the monocyte activation test (8)
MATs are not affected by components that prohibit BETs/LAL tests or RPT testing (e.g., aluminum hydroxide in vaccines, lipidic parenterals, water and dialysis solutions, and herbal components with glucan-like structures). And protocol variants exist for toxic or immunomodulatory drugs. MATs are the only type of pyrogen test for which a standardized kit version is internationally available. That system has been adapted for both solid materials and airborne pyrogens. In brief, it involves incubating a sample with cryopreserved whole human blood and quantifying a production of interleukin-1b using an enzyme-linked immunosorbent assay (ELISA) (Table 4). The cryopreserved whole blood used in this system comes from a standardized primary cell pool that has been pretested for infectious diseases and response to pyrogens. Cryopreserved blood is simply diluted after thawing without the need for washing steps.
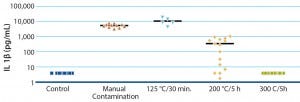
Figure 2: Pyrogen contamination and elimination from manual handling
Validated methods using cryopreserved cells are important for standardization (5). Such cells are not limited by the availability of primary cells (thus eliminating the need for blood donation on the same day) nor affected by abnormal or infected donors (through the use of safety standards during a blood transfusion). In addition, cryopreserved cells are reproducible and robust, and they perform well as predictive tools (Figure 2).
MATs represent much less than 1% of the pyrogen test market, primarily because US and European regulators have never enforced the use of accepted alternatives. In fact, between 2008 and 2011 (after acceptance of MATs), rabbit use for pyrogen testing in Europe actually increased by about 10,000 to more than 170,000 rabbits per year. That occurred despite the new EU Directive 2010/63/EU on the use of animals for scientific purposes.
In the United States (which is the primary market for pharmaceutical companies), partial acceptance by the Interagency Coordinating Committee for Validation of Alternative Methods (ICCVAM) of the whole-blood pyrogen test as only an endotoxin test has had a striking impact: The test is considered a competitor only of BETs/LAL tests, but not of the rabbit assay. That is not completely logical, because BETs/LAL tests are considered a replacement for RPTs that clearly are restricted to endotoxins, whereas there is ample evidence that the whole blood MAT covers all known human-relevant pyrogens.
Nonendotoxin Pyrogens
The relevance of nonendotoxin pyrogens (NEPs) (e.g., LTA, bacterial DNA (CpG-motif), peptidoglycan, synthetic Toll-like receptor (TLR)-agonists, lipoproteins, and endogenous pyrogens) is starting to gain significant attention, mainly as causes of human adverse reactions. Many NEPs remain unknown, however, which suggests that safety testing for pyrogenic contamination should take into account unknown pyrogenic molecules and immune system mechanisms of recognition to ensure safety of human health.
About 70% of a Gram-negative bacterial surface is covered with carbohydrate chains of LPSs, which are anchored to the outer membrane by lipophilic anchors. LTA is the Grampositive counterpart of LPS (10). Presentation of LTA on bacterial cell surfaces increases its inflammatory potency by 1,000-fold over soluble LTA (11). Therefore, the key difference between Gram-negative and Grampositive endotoxins is that the latter require presentation.
In 2013, researchers coordinating with the US Food and Drug Administration Pyrogen Working Group submitted a background review document to the ICCVAM that summarizes the available evidence for the whole-blood assay to cover nonendotoxin pyrogens such as LTA (12). The submission is waiting for an official response.
Cell-Derived Therapies and Blood-Transfusion Products
Pyrogen contamination of cell-derived therapies and blood products results in the deaths of thousands of patients every year. However, both types of products share the lack of established pyrogen testing. Although such products cannot be tested by RPTs or BETs/LAL tests, the whole-blood MAT offers a promising solution.
Preparing advanced cell-derived therapies often will require complex procedures, such as multiple cell-selection steps, ex vivo expansion, cell activation, encapsulation, and genetic modification, which all pose a risk for pyrogenic contamination. The enormous frequency of the use of blood-transfusion products also poses a considerable contamination risk. Based on 2004 data, nearly three million platelet transfusions are administered in the United States per year (1). According to recent studies with optimal culture methods of expired platelet-rich plasma, the prevalence of bacterial contamination is estimated to occur in about 1 in 750 to 1 in 1,000 platelet concentrates (1). That would suggest that 900–1,125 fatalities in the United States per year are a result of blood-platelet transfusions alone. Assuming that the average patient may receive seven platelet concentrates during a 28-day period of support, the risk of exposure to a contaminated platelet concentrate is in the range of 1 in 150 per patient.
Medical Device Testing
Medical devices are products that cross barriers within the human body. Therfore, potential contamination of such products is of great concern for patient safety. Whole-blood MATs can successfully detect pyrogens on different materials, including polystyrole, stainless steel, elastomer, and polytetrafluoroethylene (PTFE) resin. For the test, a medical device is submerged or perfused with whole human blood and monocyte activation is measured. MATs also can detect pyrogen contamination resulting from manual handling of titanium plates used for medical prostheses.
MATs have demonstrated that standard autoclaving and sterilization procedures could not completely eliminate endotoxins. Instead, such devices required incubation at 300 °C for five hours to remove the contamination.
Eliminating the Limitations
Safety testing for pyrogen contamination of pharmaceutical products and medical devices is of utmost concern for regulatory agencies and manufacturers. Neither rabbit pyrogen tests nor bacterial endotoxin tests can adequately detect the full spectrum of pyrogens. Such tests cannot be used on a number of pharmaceutical products or for the testing of solid materials such as medical devices.
The monocyte activation test is a human-cell–based assay that most closely reflects the full extent of the human immune response. The MAT overcomes the limitations of animalbased tests and offers tremendous opportunity to improve patient safety. It is the first solution that enables adequate testing of cell therapies, including blood transfusions, medical devices, and airborne pyrogens. However, the MAT has not been implemented for such applications by regulatory authorities.
Conflict of Interest Statement
Thomas Hartung holds patents as inventor of the whole blood pyrogen test and the use of cryopreserved blood, which are licensed to Merck KGaA. He receives royalties from Merck KGaA from sales of the MAT kit version.
References
1 Hartung T. The Human Whole Blood Pyrogen Test: Lessons Learned in Twenty Years. ALTEX 32(2) 2015: 79–100.
2 Nakagawa Y, Maeda H, and Murai T. Evaluation of the In Vitro Pyrogen Test System Based on Proinflammatory Cytokine Release from Human Monocytes: Comparison with a Human Whole Blood Culture Test System and with the Rabbit Pyrogen Test. Clin. Diagn. Lab. Immunol. 9(3) 2002: 588–597.
3 Martis L, et al. Aseptic Peritonitis Due to Peptidoglycan Contamination of Pharmacopoeia Standard Dialysis Solution. Lancet 365(9459) 2005: 588–594.
4 Spreitzer I, et al. Comparative Study of Rabbit Pyrogen Test and Human Whole Blood Assay on Human Serum Albumin. ALTEX 19, Suppl 1, 2002: 73–75.
5 Hartung T, et al. Novel Pyrogen Tests Based on the Human Fever Reaction: The Report and Recommendations of ECVAM Workshop 43 — European Centre for the Validation of Alternative Methods. Altern. Lab. Anim. 29(2) 2001: 99–123.
6 Dehus O, Hartung T, and Hermann C. Endotoxin Evaluation of Eleven Lipopolysaccharides By Whole Blood Assay Does Not Always Correlate with Limulus Amebocyte Lysate Assay J. Endotoxin Res. 12(3) 2006: 171–180.
7 Hoffmann S, et al. International Validation of Novel Pyrogen Tests Based on Human Monocytoid Cells J. Immunol. Meth. 298(1–2) 2005: 161–173.
8 Schindler S, et al. International Validation of Pyrogen Tests Based on Cryopreserved Human Primary Blood Cells. J. Immunol. Meth. 316(1–2) 2006: 42–51.
9 Chapter 2.6.30 Monocyte Activation Test. The European Pharmacopoeia, 6th Edition (6.7). European Pharmacopoeia Commission: Strasbourg, France, 2010.
10 Rockel C, Hartung T. Systematic Review of Membrane Components of Gram-Positive Bacteria Responsible As Pyrogens for Inducing Human Monocyte/Macrophage Cytokine Release. Front. Pharmacol. 17 April 2012: 3:56.
11 Deininger S, et al. Presentation of Lipoteichoic Acid Potentiates Its Inflammatory Activity. Immunobiol. 213(6) 2008: 519–529.
12 Hasiwa N, et al. Evidence for the Detection of Nonendotoxin Pyrogens By the Whole Blood Monocyte Activation Test ALTEX 30(2) 2013: 169–208.
Thomas Hartung, MD, PhD, is DoerenkampZbinden professor and chair of evidencebased toxicology at Johns Hopkins Bloomberg School of Public Health, joint appointment for molecular microbiology and Immunology, professor of pharmacology and toxicology at University of Konstanz, Germany, and director at Centers for Alternatives to Animal Testing (CAAT); [email protected]. Corresponding author Audrey Borel, PharmD, is global product manager, rapid release testing pharma, at Merck, Millipore SAS, Building A, 39 Route Industrielle de la Hardt – CS 49222, 67129 Molsheim Cedex, France; audrey.borel@ merckgroup.com. Gabriele Schmitz, PhD, is head of culture media and molecular biology at Merck, Merck KGaA, Frankfurter Str. 250, E060/005, 64293 Darmstadt, Germany; [email protected].
You May Also Like





