- Bioprocess Insider
- Sponsored Content

Yposkesi launches LentiSure™, LV vector production platform optimized for higher yieldsYposkesi launches LentiSure™, LV vector production platform optimized for higher yields
May 11, 2023

Sponsored by Yposkesi
New lentiviral (LV) platform provides CAR-T developers with more robust capacity to drive clinical development and commercial cell and gene therapy pipelines.
Evry-Courcouronnes (near Paris), France, April 2023 – Yposkesi, SK pharmteco’s clinical and commercial viral vector manufacturing arm for cell and gene therapies, today announces the launch of LentiSure™, an optimized Lentiviral (LV) Vector manufacturing platform for increasing lentivirus production efficiency and robustness. Lentivirus or LV vectors are used to produce cell-based immuno-oncology therapies. Their robustness as a gene delivery system is vital in determining the success of any cell-based cancer treatment.
LentiSure’s capabilities in producing LV vectors with consistent product quality, the overall average titers at cell culture harvest is at 10e7 IG/mL bioreactor volume, offer cell therapy developers the assurance of higher productivity and a higher probability of achieving the desired outcomes.
“Yposkesi is pleased to launch LentiSure, a novel and key asset able to deliver consistent yields of lentivirus vectors at the higher end of what we see on the market today,” said Alain Lamproye, chairman and CEO of Yposkesi. “This optimized LV platform is the outcome of our long-standing work in bioprocess innovation. It meets the challenges in producing cell-based immuno-oncology treatments, which is driving growth in the cell and gene therapy market, notably with the development of CAR-T cell treatments. Two new CAR-T products were approved last year in the US, with six products already on the market in both the US and Europe, along with several pending approvals. LentiSure’s scalable, high yield and high-quality lentiviral process brings net advantages to CAR-T developers and competitively positions Yposkesi as a leading European CDMO for viral vectors.”
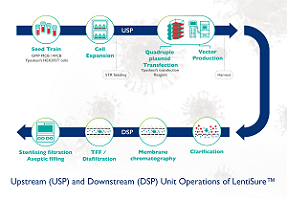
To optimize its LV platform, Yposkesi has increased efficiency at each manufacturing step. Key to this was optimizing and reducing the complexity across manufacturing unit operations, including step performance, and time and materials management. This translates into benefits in relation to client timelines, as they become more predictable for cell therapy development, and in overall costs, through optimized inputs and operations.
Lentivirus is the most commonly employed therapeutic vector (48%) across gene therapy and cell-based immuno-oncology. Oncology continues to be the leading therapeutic indication amongst all ongoing clinical trials, according to the Alliance for Regenerative Medicine, H1 2022 Report (p.10).
Yposkesi’s LentiSure LV platform is optimized to address the continued growth in cell therapy trials, which have more than doubled in number, from 181 in 2015 to 370 in 2022, and in CAR-T trials, up from roughly 50 in 2015 to 250 in 2022, according to figures from the Beacon report[1].
LentiSure, a fully integrated lentiviral vector production solution for higher yields
LentiSure provides cGMP compliant lentiviral vectors for clinical and commercial cell and gene therapy development. Its robust, scalable, and plug & play-optimized processes are designed for both adherent and suspension processes, and developed with single-use equipment. LentiSure’s main features include high titers, high transduction efficiency, reliability and scalable production processes with optimized purification techniques.
“LentiSure has already produced dozens of batches with its simplified process, designed to increase step efficiency and reduce variability. Our yields have been shown to be consistent between batches of the same product,” added Lamproye.
About Yposkesi
Yposkesi, an SK pharmteco company, is one of Europe’s largest CDMO for cell & gene therapy viral vector manufacturing. A trusted partner for companies seeking to advance clinical trials and commercialize ATMPs, Yposkesi offers a full range of services in LVV and AAV cGMP manufacturing. Within a 50,000 ft2 (soon 100,000 ft2) facility, Yposkesi operates 4 manufacturing suites for bulk DS (up to 1,000 L) and fill & finish. Yposkesi’s investment in innovation ensures that its bioprocessing platforms deliver high-quality gene-modified cell therapies and in vivo gene therapy projects.
Click here to visit Yposkesi’s website.
[1] Adoptive Cell Beacon Targeted Therapies: ‘How is the CAR-TCT landscape changing?‘ (page 8) ã Hanson Wade 2022
About the Author
You May Also Like






