Bioreactor Design for Adherent Cell Culture — The Bolt-On Bioreactor Project, Part 3: Containment, SterilityBioreactor Design for Adherent Cell Culture — The Bolt-On Bioreactor Project, Part 3: Containment, Sterility
May 12, 2015
https://bioprocessintl.com/wp-content/uploads/2015/05/13-5-Simon3_TheBoBproject.mp3
The Bolt-on Bioreactor (BoB) project is an independent initiative aimed at developing and commercializing a bioreactor for the automated and efficient culture of adherent cells, especially for application in the production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must answer four major challenges. Addressed in the first article of this series (2), the first challenge has to do with volumetric productivity. The second installment addressed issues of automating culture processes (3). This month, the third challenge is addressed. And the final installment will address process economics.
The third challenge is to develop a system in which adherent cells are cultured in a contained chamber, and where contamination risks are minimized even when the system is operated in nonclassified laboratories. This system should be provided sterile and ready to use, and when in use, it should protect cell cultures from contamination by the surrounding environment.
Contamination is a major concern in cell culture (4). Detecting biological contamination in a cell batch can turn a laboratory upside down. Unless a contamination source is clearly identified, and it can be isolated upon detection, responsible staff will want to ensure complete elimination of the contaminating agent before proceeding with further culture batches. That requires cleaning and sterilizing laboratories and equipment and (most likely) replacing consumables that could have been exposed to the contamination source.
Cell culture contamination can be physical, chemical, or biological (5). When considering our cell-culture device, we can distinguish between intrinsic contamination sources (originated in the device itself) and extrinsic sources (coming from outside the device).
Intrinsic Contamination Sources
When a culture chamber is reused, the risk of contaminating subsequent batches with the content of previous batches can be high, even after thorough cleaning of the device (6). Potential leftovers of a previous batch as well as its own unavoidable contamination can follow when cells have been harvested and the culture chamber is open and exposed to its surrounding environment.
Such potential contamination is the reason for carrying out extensive cleaning of reused equipment and setting up expensive cleaning- validation protocols. This cleaning comes with important investments in clean-in-place (CIP) and sterilization- in-place (SIP) equipment, with recurring expenses in operating costs for energy, chemicals, pure water, and labor.
Because single-use and ready-to-use cell culture devices — e.g., CellFactory (Thermo Scientific) and CellMaster roller bottle (Greiner BioOne) systems — are provided in a sterile condition, considerations regarding intrinsic sources of contamination are eliminated by using them.
Several sterilizing methods are available for cell culture devices (7). Choosing a method depends on several factors. For example, if a device is thermolabile, then autoclaving is out of the question; ethylene oxide may be an option. For plastic elements, radiation is considered to be an appropriate method, but it is not a good option for stainless steel parts because steel is a shielding material for gamma radiation (8–10).
After analyzing available sterilization methods and taking into consideration all four challenges faced by the BoB project — especially process economics — we concluded that sterilization by radiation of a compatible device is the best choice for the BoB to become standard in adherent-cell culture. Gamma irradiation is the most common sterilization method for single-use plastic devices intended for cell culture (11). Therefore, the BoB culture chamber will be provided sterile to eliminate intrinsic contamination sources. Even so, extrinsic sources of contamination remain, so the system design must help users minimize the risk of contaminating their cultures from external contamination sources. Cells should be kept within the device to prevent contamination of the laboratory or other ancillary elements used during a cell-culture process.
Extrinsic Contamination Sources
Extrinsic sources of contamination arise from one or more of the following process-related activities:
introducing or withdrawing fluids (e.g., gases or culture media) to or from the BoB culture chamber
setting up probes and sensors for measuring process parameters
moving elements that traverse the walls of the culture chamber (e.g., rotatable impeller shafts)
handling of the culture device by personnel or dedicated machines, either of which can spill contaminating agents on or neart the device to produce airborne contaminants.
Fluid Transfer: The fluid with the highest turnover rate in a culture chamber is the gaseous phase. Gas flow of 0.01 vvm in suspension cell culture is considered a very small flow rate, and dilution rate for culture medium is limited by the maximum specific growth rate of cells (12). Unless a process uses ordinary air alone, normally certified gases are purchased from a vendor, and different qualities are commercially available.
Some cell cultivators rely on a presterilized gas mixture produced in house, but usually commercial pressurized cylinders from vendors such as Air Liquide or Praxair are the gas mixture source, with a terminal sterilizing filter from Pall, Sartorius, or other supplier placed just before the gas enters or leaves a culture chamber. This is the case for Nucleo (ATMI) and Mobius CellReady (EMD Millipore) bioreactors. Terminal sterilization is a very efficient method to eliminate particulate contaminants from these gases as long as the particle size of those contaminants is larger than the filter pore size. Typically, filters with a 0.45-µm pore size are used for this purpose (13), although both larger and smaller pore-size filters are available from a number of suppliers. Pore size and filter surface need be considered because they affect pressure drop by the resistance they oppose to gas flow.
As for exhaust filters, users should take into account their potential for clogging. Venting gas has high relative humidity, creating a potential condensation risk on filter membranes. Filter clogging can cause pressure to build up within a culture chamber (14, 15). A filter heating element — such as that used by Cellbag containers (GE Healthcare) — can solve the problem. We have chosen to prevent contamination from process gases by intercalating filters of small pore-size in both inlet and outlet gas lines and using a filter heater to prevent condensation on the exhaust filter.
Commercially available capsule and cartridge filters — e.g., Fluorodyne EX filters from Pall — are specifically designed for fast-flow filtration of liquids. But dead-end disc filters are not recommended for inline sterile filtration of culture media during feeding operations because of risks posed by the pressure needed to operate such filters at required flow speeds. Consideration should be given to the undesired retention of components of interest on a filter during feeding operations as well as potential filter clogging due to cells retained during perfusion mode operation (16).
Process liquids are commonly presterilized before use and then transferred aseptically to the interior of a culture chamber as needed. Two strategies are available to prevent contamination of process liquids:
to have every needed reservoir aseptically connected to a culture chamber from the beginning (providing enough volume of culture medium and enough volume for waste/harvest)
to aseptically connect and disconnect reservoirs as they are needed throughout a culture process.
Although the second strategy is more convenient for ensuring that culture medium, waste, harvest, and other liquids used in cell culture will be stored at optimum conditions, it requires appropriate systems for aseptically connecting and disconnecting tubes. Connector standardization is a work in progress (17). But several alternatives such as the ReadyMate (GE Life Sciences), AseptiQuik (Colder Products), and Kleenpak (Pall) products are available for aseptic connection of fluid paths. A widespread standard for small-bore connections, the Luer fitting is compatible with accessories from several manufacturers (18, 19).
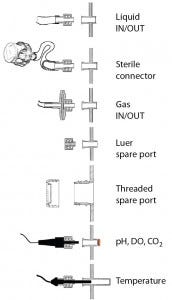
Figure 1: Default connections of fluid lines and probes to the BoB culture chamber are through Luer fittings. Spare ports for user- defined use also will be available.
The BoB team conducted a market survey on connection preferences. The vast majority of respondents chose quick connectors as the preferred option for accessories assembly (as the “Open Design Initiative” box shows). So we chose to increase the BoB’s flexibility by using Luer fittings as the default connector to its culture chamber and providing alternative accessories for connecting fluid- transfer lines. Choice of accessories will depend on the culture application and user preferences — bearing in mind that set-up will be different for products (e.g., antibodies) expressed in supernatant from that when cells are the product. A standard thread port also will be available for particular user requirements (Figure 1).
Setting Up Probes and Sensors: From a sterility/contamination point of view, inserting probes on a culture vessel is always a risky operation. Sterile, ready-to-use tubing and filters are widely available, but reusable probes require careful sterilization before use (20). Probes that measure different parameters require different sterilization and setup methods. Setting up a probe can be a delicate task when special connections are involved.
The BoB team studied available options to reduce or eliminate contamination issues related to setup of probes. First, we analyzed which control parameters are most widely used in adherent-cell culture. Then we double checked by asking the opinions of potential users regarding necessary control parameters. Appearing in a previous installment of this series (3), the results of that survey indicate that the following parameters should be measured by default: attachment- surface rotation speed and direction; medium replacement rate; thermostatting jacket temperature; incoming gas temperature and flow; gaseous phase CO2 concentration; and liquid-phase dissolved oxygen (DO), temperature, and pH. We studied available measuring methods for each of those parameters, then surveyed the market’s opinion on single-use probes (as in the “Open Design Initiative” box). Finally, we chose the measuring method that minimizes contamination risk while providing sufficient precision and accuracy for each parameter.
For all selected parameters, noninvasive measuring methods are commercially available from suppliers such as PreSens Precision Sensing, Ocean Optics, Hamilton, and others. Similar options are available for measuring other parameters (e.g., the glucose/lactate biosensor by Trace Analytics). DO, pH, and CO2 concentration can be measured using optical probes or electrodes (“optodes”), in which a small fluorescence-emitting reactive patch sends a luminous signal proportional to the analyte concentration. That luminous signal is transmitted through the transparent wall of a culture vessel and received by an optic fiber that sends a signal to the transmitter for further processing (21). The optical method is used in systems such as Wave (GE Healthcare) and UniVessel (Sartorius) bioreactors. Temperature can be measured by introducing a probe inside a pouch that prolapses from the culture- chamber wall into the inner space of the culture vessel, a so-called thermowell that allows for noninvasive temperature sensing (Figure 1).
Medium replacement rate and rotation speed and direction are both parameters that are controlled from outside the BoB culture vessel. So they do not pose a contamination threat. Finally, lid-protected spare ports will traverse the culture-chamber wall, both with standard thread and Luer taper, for users with additional requirements.
Elements That Traverse the Culture-Chamber Wall: Openings in the walls of a culture chamber present a constant contamination concern. Some such openings are unavoidable, as is the case for fluid inlets and outlets. Other openings that traditionally were necessary for measuring process parameters now can be eliminated by the use of noninvasive sensor elements. Wall openings are of concern: Openings through which a rotating or moving element traverses the chamber wall are in continuous contact with both the inside and the outside of the culture chamber and are a major concern. Such is the case when impeller shafts go through a culture- chamber wall to provide agitation within the device (22, 23).
In an effort to make such traversing shafts unnecessary, different agitation methods have been devised. We group the alternatives into three broad categories:
vessel agitation, as is used in rocking platforms with partly filled bags (e.g., Wave bioreactor systems from GE Healthcare and Biostat CultiBag systems from Sartorius Stedim Biotech) or rotating roller bottles (e.g., from Thermo Fisher or Integra Biosciences)
bubbling gas to induce mechanical agitation and aerate simultaneously (e.g., Vertical Wheel bioreactors from PBS Biotech)
magnetic agitation, such as that used to rotate impellers in some single-use bioreactors (e.g., Xcellerex XDR systems from GE Healthcare).
After thoroughly reviewing and experimenting with existing agitation methods that don’t involve moving parts traversing a culture-chamber wall, we concluded that none of them was optimum for conveying a rotational movement to the interior of the BoB’s culture chamber. We want to exclude metallic elements from within the culture chamber, we want tight control of its rotational movement, and we want a static culture chamber to which tubes and cables can be connected for continuous fluid transfer and probes while the system remains fully contained.
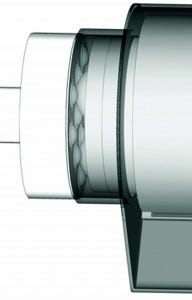
Figure 2: Movement transmission system; the rotating drive disc on the left is enclosed within the control unit (not shown) and conveys rotational movement to the driven structure by a mechanical coupling through an intercalated flexible membrane integral with the culture chamber (gray).
Following intensive design work, we developed an agitation system that fits our requirements. This system is based on mechanical coupling of plastic transfer balls (bearings) within the culture chamber to accommodate corresponding cavities between transfer balls on a driving disc outside the chamber (on the control unit). The bearings inside the culture chamber are separated from those in the control unit by a flexible membrane that provides containment without interfering with the mechanical coupling. Because the bearings inside the chamber are located in a disc that is integral to the rolled membrane on which cells attach, the rotational movement of the driving disc in the control unit conveys motion to the rolled membrane, providing for a system with nonintrusive agitation (Figure 2). An online video provides a more detailed view of this movement transmission mechanism (24).
Culture Device Handling: Personnel and machinery are both sources of contamination (5). Although that carried by laboratory technicians is typically of biological origin, contamination from equipment is most commonly related to aerosols and particles generated by heat and friction acting on lubricants and mechanical elements. In designing the BoB, we have managed to intercalate an isolating membrane between mechanical moving elements within the control unit and the working space containing the culture chamber. That makes it possible to locate control units in a room entirely separate from the cell culture laboratory, facilitating maintenance tasks and reducing contamination risks from mechanical origin.
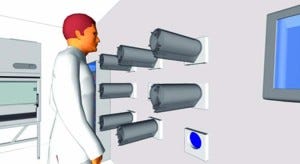
Figure 3: Multireactor unit operated from within a cell-culture laboratory with mechanical elements (control units) segregated outside the room and separated by flexible membranes (blue) intercalated within the wall. Control unit maintenance occurs outside the cell-culture laboratory.
Figure 3 shows a nine-chamber multireactor unit operated from within a laboratory with mechanical elements segregated outside the room and separated by flexible membranes intercalated in the wall. This unique BoB feature complements the increased productivity of the system to greatly reduce necessary cleanroom space in cell-culture laboratories, enabling their setup in space- constrained buildings such as hospitals.
As for human sources of contamination, most critical BoB operations are those in which the culture chamber’s inner space (or its media-containing reservoirs) becomes exposed to a laboratory environment. That typically happens during bioreactor setup (at the start of a culture process) and during operation whenever tubes are connected or disconnected. Probes connection is also a risky operation. By reducing the number of necessary tube connections and disconnections, we also reduce contamination opportunities. That is achieved through automation and continuous (perfusion) cell culture (2, 3). But for occasions in which manual manipulation cannot be avoided, we must rely on design features described above.
References
1 Bolt-on Bioreactor: Automated Adherent Cell Culture, 2014; www.boltonbioreactor.com.
2 Simon M. Bioreactor Design for Adherent Cell Culture: The Bolt-on Bioreactor Project, Part 1 — Volumetric Productivity. BioProcess Int. 13(1) 2015: 28–33.
3 Simon M. Bioreactor Design for Adherent Cell Culture: The Bolt-on Bioreactor Project, Part 2: Process Automation. BioProcess Int. 13(4) 2015: 40–48.
4 Stacey GN. Cell Culture Contamination. Methods Mol. Biol. 731, 2011: 79–91.
5 Lincoln KK, Gabridge MG. Cell Culture Contamination: Sources, Consequences, Prevention, and Elimination. Animal Cell Culture Methods. Academic Press: San Diego, CA, 1998.
6 Mollah AH, White EK. A Risk Based Cleaning Validation in Biopharmaceutical API Manufacturing. BioPharm Int. 18(11) 2005: 54–67.
7 Rutala WA, Weber DJ, and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Centers for Disease Control and Prevention: Atlanta, GA, 2008.
8 Half-Value Layer (Shielding). NDE Resource Center; https://www.nde-ed.org/ EducationResources/CommunityCollege/ RadiationSafety/safe_use/shielding.htm.
9 Radiation Safety Manual. Stanford University Environmental Health and Safety: Stanford, CA, March 2010 (updated January 2015); http://web.stanford.edu/dept/EHS/ prod/researchlab/radlaser/manual/rad_safety_ manual.pdf.
10 Procedure 17: Shielding. University of New Brunswick: Fredericton, NB, Canada. www.unb.ca/fredericton/environmental- safety/_resources/pdf/safety-manual/ procedures/shielding.pdf.
11 Niazi SK. Disposable Bioreactors. Disposable Bioprocessing Systems. CRC Press: Abingdon, UK, 2012.
12 Lubiniecki AS. Large-Scale Mammalian Cell Culture Technology. Dekker: New York, NY, 1990.
13 Doran PM. Bioprocess Engineering Principles. Academic Press: Waltham, MA, 2013.
14 Flickinger MC. Downstream Industrial Biotechnology: Recovery and Purification. John Wiley & Sons: Hoboken, NJ, 2013.
15 Jornitz MJ, Meltzer TH. Filtration and Purification in the Biopharmaceutical Industry, 2nd Edition. Informa Medical: London, UK, 2008.
16 Chotteau V. Perfusion Processes. Animal Cell Culture. Al-Rubeai M, Ed. Springer: New York, NY, 2015.
17 Martin J. Focus on Standardization, Quality by Design, and Regulatory GMP. BioPharm Int. November 2011.
18 ISO 594-1:1986. Conical Fittings with a 6% (Luer) Taper for Syringes, Needles, and Certain Other Medical Equipment — Part 1: General Requirements. International Organization for Standardization: Geneva, Switzerland, 1986.
19 ISO 594-2:1998. Conical Fittings with 6% (Luer) Taper for Syringes, Needles, and Certain Other Medical Equipment — Part 2: Lock Fittings. International Organization for Standardization: Geneva, Switzerland, 1998.
20 Sensors for Monitoring and Control. Monitoring and Control of Fermenters. Montague G, Ed. Institution of Chemical Engineers: London, UK, 1997.
21 Scheidle M, et al. Combination of On-line pH and Oxygen Transfer Rate Measurement in Shake Flasks By Fiber Optical Technique and Respiration Activity Monitoring System (RAMOS). Sensors 7, 2007: 3472–3480.
22 Sikyta B. Techniques in Applied Microbiology. Progress in Industrial Microbiology. Elsevier: Amsterdam, The Netherlands, 1995.
23 Ozturk SS. Equipment for Large-Scale Mammalian Cell Culture. Mammalian Cell Cultures for Biologics Manufacturing. Zhou W, Kantardjieff A, Eds. Springer, NY, 2014.
24 Challenge #3. Bolt-on Bioreactor: Automated Adherent Cell Culture, 2014; www. boltonbioreactor.com/the-bob/the-four- challenges/generica_3.html.
Marcos Simón, PhD, is founder of the Bolt- on Bioreactor Project, Technology Park, Mikeletegi 56, 20009 San Sebastian, Spain; [email protected]; www.boltonbioreactor.com.
You May Also Like





