New Bioprocessing Standards from Molecule to MarketNew Bioprocessing Standards from Molecule to Market
July 1, 2010

Life Technologies is at the forefront of a quest to improve the human condition. Growing numbers of biotherapeutics, revolutionary blood products, emerging biosimilars, and a vaccine industry moving toward cell culture–based production systems all promise to transform traditional disease treatment.
Leveraging core expertise in cell culture, downstream purification, and rapid molecular-based contaminant testing, life technologies offers biotherapeutic and vaccine production solutions under trusted GIBCO® and Applied Biosystems® Brands.
GIBCO® Media and Feeds for Upstream Cell Culture
New in 2010, GIBCO® CD FortiCHO, is a fortified, protein-free, animal origin-free medium for recombinant CHO cells designed to deliver better cell productivity, better cell growth, and longer culture duration. GIBCO® PD-direct Services continue to help with process optimization, spent media analysis and rapid prototyping.
POROS® Chromatography Resins for Downstream Purification
POROS® chromatography resins are today’s best-performing media for process-scale bioseparations. The new POROS® XS resin is the first high-capacity, high-resolution resin that allows you to load in the presence of up to 15 mS/cm salt, more than 100-mg/ml capacity, while delivering unprecedented impurity clearance. The GoPure™ Prepacked Column combines connect-and-go convenience with high performance POROS® resins. The system offers easy, ready-to-connect columns that speed manufacturing-suite turnaround and eliminate cross contamination.
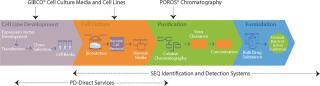
SEQ Identification and Detection Analytics
SEQ Rapid Molecular Methods help detect impurities and identify contaminants by using molecular techniques, such as DNA sequencing, PCR and real-time PCR, and are fast becoming the standards for pharmaceutical analytics worldwide. The Cell Culture rapid Methods Program can screen for Mycoplasma, Vesivirus and Mouse Minute Virus (MMV) contamination using only one sample preparation and one instrument platform, in less than four hours. the resDNASEQ™ CHO residual DNA Quantitation System uses qPCR to measure host cell residual DNA levels in under five hours with unprecedented sensitivity and specificity.
About the Author
Author Details
Rachel Goodrich is with Life Technologies Corporation, 5781 Van Allen Way, Carlsbad, CA 92008; 1-760-603-7200; [email protected], www.lifetech.com/bioprocessing.
You May Also Like






