The Crossroads of Academia, Industry, and Education: Modern Training Centers Are Pivotal to the Future of R&DThe Crossroads of Academia, Industry, and Education: Modern Training Centers Are Pivotal to the Future of R&D

The Australian Research Council (ARC) Training Centre for Biopharmaceutical Innovation (CBI) at the University of Queensland in Brisbane, Australia
Global pharmaceutical industry research and development (R&D) investment has experienced steady growth over the past two decades, with an anticipated compound annual growth rate (CAGR) of 3.0% and projected 2024 investment of US$213 billion (1). Focused on developing innovative therapies for chronic, infectious, genetic, and lifestyle-related ailments, the fast-growing biologics segment has become a cornerstone of the pharmaceutical industry and healthcare sector. The demonstrated effectiveness and wide-ranging applicability of biopharmaceuticals also have brought considerable R&D in computational and biological technologies. Although such critical investments are contributing to an increased number of drugs in clinical trials, companies also are realizing the need to invest in other areas such as the future workforce.
Historically, the worlds of academia and industry have been isolated from each other. Companies, universities, and institutions collaborate, but such work is often restrictive and underexploits the strengths of all parties involved. That hinders the potential for scientific advancement and the training needed to create the well-rounded, experienced leaders of tomorrow. Herein, we highlight an Australian training center that facilitates the cooperation of industry and academic partners with a strong emphasis on developing a skilled workforce for the biopharmaceutical sector, accelerating a graduate’s ability to contribute to the field.
The Australian Research Council
(ARC) Training Centre for Biopharmaceutical Innovation (CBI) was established in 2017 to foster close collaborations between university-based researchers and Australia’s growing biopharmaceutical industry. CBI combines the efforts and support of the Australian government, the University of Queensland in Brisbane, the Pharma Services Group of Thermo Fisher Scientific, Cytiva, CSL Behring, and the Australian Red Cross Blood Service in three thematic areas: discovery, development, and advanced manufacturing. CBI also benefits from close collaboration and colocalization with Australia’s National Biologics Facility (2).
Industry partners are critical components of CBI and contribute to the effective supervision, on-site training, and mentoring of doctoral students and postdoctoral researchers. With our industry partners at Thermo Fisher Scientific and Cytiva, the advanced manufacturing research team focuses on improvements in continuous upstream and downstream bioprocess efficiency.
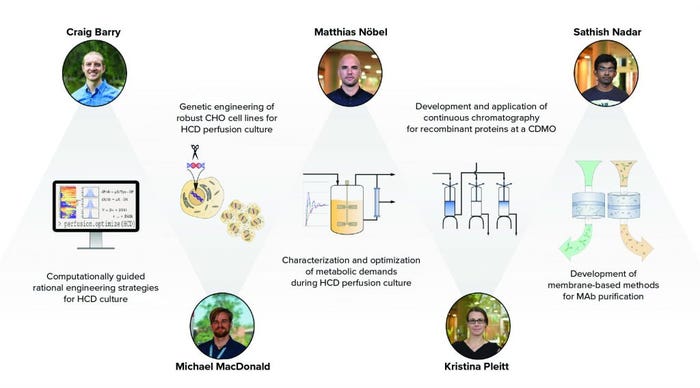
Figure 1: CBI participants undertake research and industry placements that span the biotherapeutic production process; HCD = high cell density, CHO = Chinese hamster ovary, CDMO = contract development and manufacturing organization, and MAb = monoclonal antibody.
Student Success
The sidebars herein chronicle the experiences of five CBI students who work with Thermo Fisher Scientific– and Cytiva-based projects. The students describe lessons that they have learned during their doctoral programs.
From a technical perspective, these five students share a connection through their projects, which together span a nearly complete overview of the biotherapeutic production process (Figure 1). The connectedness of these projects facilitates cohesion among the students, all of whom can seek advice from their peers. In addition to working with respected peers, students have advisors from both academic and industrial backgrounds who present their own viewpoints and deliverables.
Altogether, CBI provides a diverse and dynamic array of professional and technical challenges that stimulate students to interact across gaps in experience, technical focus, professional objectives, and even physical location. Such challenges create opportunities for development and growth that are not possible in traditional university courses and industry placements.
Craig Barry, Computational Modeling |
|---|
During my undergraduate studies in chemical engineering and for a short stint in industry, I largely gained exposure to the world of inorganic commodity chemicals. Working as an engineer, I developed an interest in dynamic modeling for design and optimization. I liked the idea of mathematical modeling for rational design of industrial cell lines and bioprocess unit operations. That quickly grew into a passion during my postgraduate research on tetanus vaccine bioprocess optimization at the University of Queensland. Substantial improvements to biopharmaceutical cell-culture technologies require intelligent engineering of robust cell lines that are suited to unique bioreactor environments. Using a proteomic-centered framework, my project aims to build a computational model that investigates the biological mechanisms of Chinese hamster ovary (CHO) cell proliferation and programmed cell death. Dynamic modeling is used to give order to complex data sets and to account for noise, which is inherent to cellular biology. Ultimately, in silico interrogation of a model offers a means to experiment with rewired CHO cell biology, enabling future development of more robust manufacturing strains. CBI greatly facilitated my ability to exercise my passion in the biopharmaceutical industry through an engaging doctoral project with Thermo Fisher Scientific. With the company’s guidance and exposure to the biologics industry, I have gained perspective on what the near future may hold for that industry and how I can play a part in it as an engineer. I’m eager to gain industry experience through an internship with Thermo Fisher Scientific. I thoroughly enjoy the continual academic challenges of a PhD project and the freedom of thought that the project facilitates. |
Michael MacDonald, Cell Line Development |
|---|
Cell death often is considered to be inevitable and unpredictable in upstream production, but it is controlled by deliberate decision-making signaling pathways within cells, most notably during apoptosis. My CBI colleagues and I have published a review describing the work in this area (3). My project focuses on using CRISPR technologies to incorporate rational modification of critical points in apoptotic pathways to create hardy cell lines that are resistant to process stress. The ultimate goal is to develop cells that live longer and are more productive and efficient than currently available cell lines, reducing media costs and increasing titer. I am eager to find a niche between the theoretical and practical aspects of the life sciences. Having worked as both an academic researcher and a production scientist, I considered the opportunity to combine these two aspects in a doctoral setting to be an ideal first step into R&D. In addition to providing technical knowledge and experience with the latest technologies, working in the space between academia and industry has enabled me to encounter and negotiate unique challenges. Through those challenges and with the support of mentors from many walks of life, I have cultivated a skill set that will prove invaluable in my future endeavors. CBI also has enabled me to work in different physical and professional spaces and to meet and work with groups of amazing people. Over the course of my doctoral program, I have been surprised routinely by the diversity and skills of the people with whom I work. I believe that it was a tremendous insight by the ARC and Thermo Fisher Scientific to commit to this center and that their investments will yield promising results. |
Matthias Nöbel, Perfusion Culture |
|---|
High cell densities often are linked directly to increased overall process productivity. Because of single-use technologies and improved process monitoring tools, continuous perfusion systems are gaining in popularity. Compared with traditional discontinuous operations, increased cell densities and changes in cell environments are making new metabolic demands of perfusion cell systems. My project uses a systems-biology approach to analyze cellular proteins and metabolites under different conditions to understand metabolic changes. Ideally, my findings will be translated into approaches that maximize the metabolic performance, stability, and product yield of perfusion culture systems. Unlike other CBI entrants, my experience with the biopharmaceutical industry was limited. Having the opportunity to explore beyond an academic environment and gain industry insight was one of the major draws for me to join the center. For my industry placement, I spent time at Thermo Fisher Scientific’s R&D facility in Princeton, NJ, which specializes in manufacturing a wide range of biopharmaceutical proteins. From the start, I learned about the differences between academia and industry. Starting with the size of teams and operational units and stretching to the work structure itself, my activities at Thermo Fisher Scientific differed substantially from my work at a university. Because of my placement, now I understand the distinctive challenges faced in a commercial environment: documentation, scale-up, and simultaneous management of departments, customers, and colleagues. The guidance of experts in those fields helped me develop skills I needed to solve new problems quickly and effectively.The perspective I am gaining makes me feel well prepared for what lies ahead of me. My daily work at the university has gained a boost as well thanks to the techniques and structures I learned during industry placement. |
Kristina Pleitt, Continuous Chromatography |
|---|
Market pressure for fast, cost-effective production of clinical material has increased outsourcing to contract development and manufacturing organizations (CDMOs). To meet client expectations, CDMOs seek to offer robust approaches for development, production, and testing of products with competitive pricing and timelines. My project focuses on the potential of continuous downstream processing for improving manufacturing efficiency and flexibility. Using process-simulation software, I determined the most favorable situations for implementing continuous and semicontinuous downstream processing for monoclonal antibodies (MAbs) (4). The common thread among MAb downstream platforms is product capture using protein A, which makes conversion from batch to continuous operation relatively straightforward. But CDMOs work with a wide range of proteins, and without a suitable affinity resin, purification is challenging. I am investigating continuous processing strategies for non-MAb recombinant proteins. After my undergraduate work, I joined a process development group at a newly formed CDMO. I was learning constantly and enjoyed the work. It is rewarding knowing that your work contributes to patients’ receiving the treatments they need. I could not imagine stepping away from my job, but I was interested in pursuing a doctoral degree and knew that doing so would help my career. When a former manager told me about CBI, I jumped at the opportunity. CBI’s partnership between academia and industry allows me to remain active in both areas. More important, the research is relevant and something that I want to study.I’ve grown personally and professionally through mentorship from CBI’s researchers and industry partners. I appreciate how CBI projects are focused on issues that are applicable to the biopharmaceutical industry and how the program emphasizes strong research principles as well as several “soft skills.” It can be challenging to balance full-time doctoral work with a full-time job, but I’m fortunate to have a supportive network through CBI and my company, Thermo Fisher Scientific. I’m thankful to work in such a rewarding field. |
Sathish Nadar, Membrane Technology |
|---|
The biopharmaceutical industry has been conventional in its approach to developing production processes for recombinant therapeutic drugs, mainly because of a highly regulated environment. However, surges in demand for this class of molecules and the advent of biosimilars have forced the industry to seek out innovative and cost-effective solutions to remain competitive. My ambition is to help find beneficial solutions for both patients and the industry. Biologics manufacturers traditionally have used resin-based chromatography to purify protein therapeutics. Despite being a powerful technology, resins are expensive, work slowly, and require elaborate cleaning and associated validation. Because of its faster operation and its availability in single-use options, membrane technology represents an alternative to resin-based chromatography. My doctoral project assesses the performance and potential cost benefits of membrane-chromatography processes. I was introduced to CBI by a colleague at my past organization who had joined the program as a postdoctoral researcher. At that point, I was not sure of taking up this PhD program because for me it meant leaving a comfort zone within a well-organized R&D department in a multinational biopharmaceutical industry and travelling 6,000 miles away from my hometown. However, after working for four years in bioprocess R&D, I started to feel a stagnancy in terms of knowledge and an urge to take up something more challenging. It did not take long to convince myself to undertake doctoral study, which I would rather call my “big promotion.” At CBI, I still feel that I am part of a well-structured industry project mentored by highly experienced leaders, but now I am the one making the critical decisions and getting the opportunity to learn both technical and project- management skills. I do sometimes feel exposed and challenged in front of the vast experience of industry mentors, but at the same time, I am protected when my academic supervisor guides me through difficult times in my project and helps me learn from my mistakes. Currently in the second year of my degree program, I have no regrets about leaving a job for this program because this journey has been both enriching and exciting. |
Values and Strengths
CBI students benefit from a special position at the intersection of academia and industry at a time of critical professional development. Such positioning forms a crucible in which students are allowed, supported, and encouraged to thrive in ways that become individualized to their strengths and weaknesses, doubts and ambitions. Fostering their distinctive skill sets helps them to develop tools for future success in any sectors that they pursue.
Innovation: The freedom of CBI’s academic environment in combination with the watchful eyes of experts from industry enables students to take risks, innovate, and grow as professionals. Such skills are essential to the success of R&D and will be highly sought after.
Collaboration: By working in a professionally and culturally diverse environment, which is central to CBI’s philosophy, students learn to collaborate across hierarchical, technical, and social boundaries to succeed in their projects. That skill is valuable to the increasingly multidisciplinary and international nature of biopharmaceutical R&D.
Leadership: During a doctoral program, students become subject-matter experts. Because of the broad scope and flexibility of their projects, the diverse needs of their many stakeholders, and their access to expertise and resources from interdisciplinary advisors, CBI students develop the leadership skills necessary to unify such factors under a single banner: the thesis. The need to communicate and negotiate with the many parties involved in a student’s doctoral journey is an unparalleled training ground for the rigors of a future career at the forefront of science and commerce.
Project Management: During their studies, doctoral candidates transition gradually from student to master. With projects that take place across technical and professional environments, CBI students develop project management skills that are tailored to different situations. Industrial placements allow students to travel and experience different environments, which broadens their horizons and helps them develop invaluable professional networks. That ultimately creates graduates who can take on important responsibilities at different stages in the drug pipeline and in different locations across the globe.
Resilience: Fulfilling doctoral requirements is a professionally and technically demanding process, and one of CBI’s strengths is to ensure that students undergo this process with support networks for both their current and future endeavors. The center’s commitment to their projects further prepares students to adapt to the ever-changing needs of commercial R&D.
Sustainable Growth for Aspiring Professionals
Kym Baker (general manager at Thermo Fisher Scientific–Brisbane) says that her company is delighted to support ARC as an industry partner. “We see real value in partnering with academia to create a sustainable growth platform for the aspiring scientists and engineers of tomorrow by providing them with real-life projects and challenges [that] complement academic learning with experiential development.” Baker adds, “With both industry and academic feedback sessions, the projects place strong emphasis on current industry trends, thereby preparing students to transition readily to the industry upon completion of their respective degrees.”
Stephen Mahler (CBI director and senior group leader at the Australian Institute for Bioengineering and Nanotechnology) says, “It has been a rewarding experience to observe how much our doctoral students have benefited from real-world interactions and experiences with our industry partners.” Reflecting on the future of biologics R&D, Mahler notes, “It is imperative that universities and industry work more closely together [than they have in the past] to provide streamlined career pathways and transitions into industry for our PhD graduates — and to provide ‘industry ready’ graduates for the rapidly expanding bioeconomy. Success for CBI will be measured by following our graduates’ career paths and appreciating their collective contributions.”
By combining the strengths of commercial and academic R&D, CBI provides students with novel challenges and support systems that they need to develop into highly valued professionals for the future of both sectors. Furthermore, collaborations between academia and industry on neutral grounds promote a blurring of the lines between the two traditionally isolated fields to enable meaningful research. In these endeavors, research and development applies to technology and to people, because students both learn and contribute to this important and ever-growing field.
References
1 World Preview 2019: Outlook to 2024. EvaluatePharma: London, UK, June 2019; https://www.evaluate.com/thought-leadership/pharma/evaluatepharma-world-preview-2019-outlook-2024.
2 Munro T, et al. Bridging the Gap: Facilities and Technologies for Development of Early Stage Therapeutic MAb Candidates. mAbs 3(5) 2011: 440–452; https://doi.org/10.4161/mabs.3.5.16968.
3 Henry M, et al. Attenuating Apoptosis in Chinese Hamster Ovary Cells for Improved Biopharmaceutical Production. Bioeng. Biotechnol. 117(4) 2020: 1187–1203; https://doi.org/10.1002/bit.27269.
4 Pleitt K, et al. Evaluation of Process Simulation as a Decisional Tool for Biopharmaceutical Contract Development and Manufacturing Organizations. Biochem. Eng. J. 150, 15 October 2019: 107252; https://doi.org/10.1016/j.bej.2019.107252.
Craig Barry, Michael MacDonald, Sathish Nadar, Matthias Nöbel, and Kristina Pleitt are doctoral students; Gary Shooter is a postdoctoral research fellow of downstream biopharmaceutical projects; and corresponding author Verónica S. Martínez is a postdoctoral research fellow of upstream biopharmaceutical projects, all at the ARC Training Centre for Biopharmaceutical Innnovation, University of Queensland, Brisbane QLD 4072, Australia; [email protected].
You May Also Like





