Cell-Line Development for Expressing IgM AntibodiesCell-Line Development for Expressing IgM Antibodies
The polyvalency of IgM molecules gives them high-avidity binding to their targets. HTTPS://IGMBIO.COM
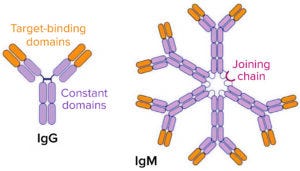
Figure 1: Compare the structures of IgG and IgM antibodies.
Immunoglobulin G (IgG) antibodies have been studied and applied as biopharmaceuticals for decades, and they remain dominant in the monoclonal antibody (MAb) pipeline. By contrast, immunoglobulin M (IgM) molecules are much larger and consequently more challenging for biomanufacturing and therapeutic application. Essentially, they appear as clusters of the familiar Y-shaped IgG molecules, joined at their bases in pentameric (Figure 1) or hexameric forms. That structure gives them 10 and 12 binding moieties, respectively, which translate to superior binding power (avidity) of the antibodies compared with IgG isomers.
IGM Biosciences of Mountain View, CA, believes that IgMs could be more effective than IgGs at treating certain diseases — in particular as cancer immunotherapies. Many researchers in the past have been stymied by the technical barriers to IgM manufacture. But over the past 10 years, IGM scientists and engineers have made a series of breakthroughs that now make the company the only developer that can produce engineered IgM antibodies efficiently at g/L scale — in its dedicated good manufacturing practice (GMP)–compliant facility.
Christina Pingchuan Tsai has been the senior scientist leading IGM’s cell-line development group since 2021. With eight years of industry experience focusing on development of cell lines and biologics production processes at Samsung Biologics and Hengenix Biotech, she specializes in high-throughput cell-culture screening and purification and cell-line characterization using proteomics/genomic assays and next-generation sequencing (NGS). Previously she spent five years in the microbiome therapeutics industry with a similar focus on NGS profiling and assay development as well as sample preservation and bacteriophage production.
With a 2003 bachelor of science degree in chemistry from Taiwan’s National Tsing Hua University and a 2009 PhD in biological chemistry from Texas A&M University, Tsai did postdoctoral work at Stanford University in 2010–2012. There, she developed protein-characterization assays and studied bi-/multispecific antibodies targeting cancer cells on a fellowship awarded by the US National Institutes of Health’s cancer biology program.
BPI West Presentation
Tsai’s presentation at the BioProcess International US West Conference and Exhibition in San Diego, CA (March 2022), was titled “Cell-Line Characterization for IgM Molecules in Cell-Line Development Process.” In it, she highlighted the importance of cellular, metabolic, proteomic, and genomic studies. She demonstrated that clinical results show the greater potency of IgMs over IgGs, as demonstrated by stronger and more physiologic binding for improved control of T-cell activation. Her company has worked to convert IgGs to IgM structures for the increased avidity, then engineered those molecules for improved specificity and extended in vivo half-life, and finally created bispecific antibody formats.
Tsai summarized her group’s cell-line development workflow as follows:
• transfection of a selected pool of cells to produce monoclones
• screening for outgrowth of monoclones
• narrowing down top clones by titer, growth, and protein-quality evaluation.
• testing small amounts of top clones for stability (in bioreactors).
The cell line with highest stability and specific productivities is selected as a final lead clone. Cell lines are characterized for their growth and productivity, for the quality of proteins they express (the pentameric form is preferred), and for their viability and behavior in bioreactor cultures. Genomic characterization is used to ensure transgene integrity and evaluate mutations, with the ultimate clones further tested for their growth and stability. Potency and ELISA assays help determine the quality of the antibodies produced.
Our Conversation
Before we met at BPI West, I chatted with Tsai virtually about the subject of her talk there.
What is the main appeal of IgMs as potential biotherapeutics? Can you tell us a bit about their binding power and strengths compared with those of the more familiar IgGs? An IgM has 10 arms that can bind to target antigens, whereas an IgG has only two arms. The additional binding sites provide superior binding power (enhanced binding avidity).
The IGM-2323 molecule in our clinical phase 2 program has 10 binding sites for the B-lymphocyte antigen CD20 on cancer cells and one binding site for the CD3 protein complex on T cells, so it can engage with cancer cells and attract T cells to kill them. This novel bispecific IgM antibody has potent activity through T-cell directed cytotoxicity (TDCC) and retains the robust complement-dependent cytotoxicity (CDC) activity characteristic of IgM antibodies. In our preclinical studies, it also has shown reduced cytokine release compared with IgGs. That could account for the low incidence of adverse reactions reported during phase 1 clinical trials (1).
What technical challenges arise in development and manufacturing of IgM antibodies? Some researchers have reported solubility limitations and a tendency for IgMs to denature in processing (2–4). And how does your company tackle those difficulties? The main technical challenge of developing and manufacturing IgM molecules is to obtain the right format. IgM has pentameric and hexametric forms, and we are mainly focusing on development of the pentameric form that contains an open J-chain. And in IgM purification, we cannot use protein A to separate the antibodies from impurities in cell-culture supernatants because IgM does not contain the protein A binding motif. We have developed proprietary processes to separate IgM molecules with high purity.
Shifting to cell-line development specifically, BPI published a report on IgM expression in PER.C6 cells about 10 years ago (5). What cell line(s) do you use for IgM production? Do they present any special challenges? We use typical cell lines that generate biologics. They are neither difficult to work with nor a challenge to characterize for IgM production. We must make sure that the cell lines are producing the pentameric IgM molecule that matches our quality considerations.
The IgM pentameric structure looks like a ring of five IgGs connected by disulfide bonds. Are those five moieties typically repeats of one another, or does each one have its own binding target? Our IgM molecules have a repeated binding motif on five moieties. Some of our molecules are bispecific, with another binding motif on their J-chains.
Does the large size of IgMs (~900+ kDa) translate to a much larger gene sequence than that for IgGs (~150 kDa), and would that complicate the generation/integration of IgM expression vectors? The plasmids for IgM generation contain genes that encode for heavy chains, light chains, and J-chains. There are not too many differences between those and the plasmids for IgG. We have not seen difficulties in plasmid generation and genetic integration for IgM generation. which can be larger than the typical plasmids for IgG expression.
In your presentation at BPI West, you highlighted cell-line and product characterization methods. What techniques do you use? My presentation mainly focuses on characterization technologies for clone selection. We use sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), ELISA, potency assays, bioreactor screening, and stability studies. The assays are important for characterizing the cell lines that can produce IgM with the best quality at ideal productivities. Bioreactor screening is used to investigate metabolites from different clones. Cell-age stability studies are critical to determining whether selected cell lines can produce IgMs at comparable productivities as cell age increases, considering the number of generations needed to get to scale.
Protein A purification cannot be used, and although other affinity chromatography resins are commercially available and useful for research-scale purification, they currently are not scalable (6). So we use different types of chromatography for IgM purification.
Why do you focus on the J-chain in IgM product quality assessments? J-chain is one of the critical components to the formation of pentameric IgM molecules. Other forms of IgM can still be expressed if the J-chain is missing. IGM-2323 in our phase 2 clinical trial does contain a CD3-binding motif on its J-chain. In some of our characterization assays, we measure the relative amounts of J-chain from clones, which are critical for T-cell activation.
Timelines are increasingly important in cell-line development. Automation technologies have helped many IgG developers speed up their engineering and selection processes. Are you able to do the same with your IgM processes? Yes, we are building our automation capabilities to improve our clone-selection processes. This technology helps us screen more clones with high expression titers and stability.
References
1 A Safety and Pharmacokinetic Study of IGM-2323 in Subjects with Relapsed/Refractory Non-Hodgkin Lymphoma. IGM Biosciences, Inc.: Mountain View, CA; https://clinicaltrials.gov/ct2/show/NCT04082936.
2 Chromikova V, et al. Evaluating the Bottlenecks of Recombinant IgM Production in Mammalian Cells. Cytotechnol. 67(2) 2015: 343–356; https://doi.org/10.1007/s10616-014-9693-4.
3 Gagnon P, Hensel F, Richieri R. Purification of IgM Monoclonal Antibodies. BioPharm Int. March 2008; https://www.biopharminternational.com/view/purification-igm-monoclonal-antibodies.
4 Mader A, Chromikova V, Kunert R. Recombinant IgM Expression in Mammalian Cells: A Target Protein Challenging Biotechnological Production. Adv. Biosci. Biotechnol. 4(4A) 2013: 30616; https://doi.org/10.4236/abb.2013.44A006.
5 Valasek C, et al. Production and Purification of a PER.C6-Expressed IgM Antibody Therapeutic. BioProcess Int. 9(11) 2011: 28–37; https://bioprocessintl.com/downstream-processing/separation-purification/production-and-purification-of-a-per-c6-expressed-igm-antibody-therapeutic-324813.
6 Keyt BA, et al. Structure, Function, and Therapeutic Use of IgM Antibodies. Antibodies9(4) 2020: 53; https://doi.org/10.3390/antib9040053.
Further Reading
Carroll S. An IgM Antibody Platform to Tackle SARS-CoV-2 and Its Variants. Drug Target Rev. 23 September 2021; https://www.drugtargetreview.com/article/97106/an-igm-antibody-platform-to-tackle-sars-cov-2-and-its-variants/2.
Hennicke J, et al. Transient Pentameric IgM Fulfill Biological Function: Effect of Expression Host and Transfection on IgM Properties. PLOS One 15(3) 2020: e0229992; https://doi.org/10.1371/journal.pone.0229992.
Ku Z, et al. Nasal Delivery of an IgM Offers Broad Protection from SARS-CoV-2 Variants. Nature 595, 29 July 2021: 718–723; https://doi.org/10.1038/s41586-021-03673-2.
Matsuda Y, Imamura R, Takahara S. Evaluation of Antigen-Specific IgM and IgG Production During an In Vitro Peripheral Blood Mononuclear Cell Culture Assay. Front. Immunol. 10 July 2017; https://doi.org/10.3389/fimmu.2017.00794.
Trommet V. Master Thesis: Production and Characterization of IgM Antibodies. University of Natural Resources and Life Sciences Department of Biotechnology: Vienna, Austria, November 2019; https://abstracts.boku.ac.at/download.php?dataset_id=19437&property_id=107.
Wang BT, et al. Multimeric Anti-DR5 IgM Agonist Antibody IGM-8444 Is a Potent Inducer of Cancer Cell Apoptosis and Synergizes with Chemotherapy and BCL-2 Inhibitor ABT-199. Mol. Cancer Ther. 20(12) 2021: 2483–2494; https://doi.org/10.1158/1535-7163.MCT-20-1132.
Cheryl Scott is cofounder and senior technical editor of BioProcess International, part of Informa Connect; 1-212-600-3429; [email protected]. BPI associate editor Brian Gazaille was instrumental in coordinating this conversation.
You May Also Like





