Addressing Unwanted Immunogenicity in Gene TherapiesAddressing Unwanted Immunogenicity in Gene Therapies
Immunogenicity is the ability of a substance, such as a foreign and/or potentially dangerous protein, to provoke an antigen-specific immune response. However, some immune responses can be detrimental, such as in autoimmune diseases and unwanted reactions to biological therapeutics. The latter case can compromise biopharmaceutical safety and efficacy, and preexisting immunity against biologic components can preclude patients from receiving life-changing treatments, perhaps most notably in gene therapy (1).
Gene therapies are designed to target the root cause of a genetic disease by gene replacement, addition, inhibition, or editing (2). Often, such treatments are delivered by viral vectors. The current standard for in vivo gene delivery is adenoassociated virus (AAV), which was detected in humans in the 1960s (3). AAV is the most widely applied gene therapy vector for several reasons. It can transduce host cells and shows robust, long-term transgene expression, but it is largely unable to integrate into the human genome (4–6). AAV vectors also are highly amenable to genetic engineering of their cargo and capsids (7), enabling selective tropism and increasing potential for treatment success across the broad landscape of genetic diseases.
Despite such advantages, difficulties remain. One of the largest hurdles is vector immunogenicity. Serious issues have occurred when high vector doses are administered, yet such doses sometimes are necessary to ensure therapeutic efficacy under the current one-time-only treatment paradigm. Upon administration, vectors are detected by innate immune receptors, such as toll-like receptors (TLRs). This activation can cause inflammation and trigger adaptive immune responses from antigen-specific
T and B cells, producing neutralizing antibodies (NAbs) that preclude subsequent dosing of that vector (8). As many as 40% of patients who could benefit from gene therapies are ineligible for treatment because they have preexisting NAbs from prior infections with naturally occurring AAVs (9). Moreover, T cells can identify and eliminate AAV-transduced cells, diminishing therapy durability and causing liver inflammation (10). Addressing both preexisting and de novo immunogenicity hurdles will be critical to unlocking the full potential of life-saving therapies for patients with significant unmet need.
Current Strategies for Mitigating AAV Immunogenicity
Risks for AAV immunogenicity generally increase with dose (11, 12). Even at moderate doses of 2 × 1012 viral genomes (vg) per kilogram of patient weight, hemophilia B patients have shown increases in liver enzyme activity that correlate with the appearance of capsid-specific T cells. However, treating patients with low doses of a one-time-only therapy is risky. If the dose is too low, the therapy will be ineffective and the patient’s chance to be treated by the gene therapy will have been wasted. Anti-AAV NAbs and cross-reactive antibodies that form in response to low-to-moderate vector doses of 8 × 1010 vg/kg to 2 × 1012 vg/kg can persist for up to 15 years.
Except for some hemophilia treatments, most gene therapies are administered at doses of 5 × 1012 vg/kg or higher for liver-based diseases and 1014 vg/kg or higher for neuromuscular diseases. A dose of 1–3 × 1014 vg/kg represents a tremendous capsid burden. By contrast, the average adult has ~37 billion cells. Thus, high vector doses can result in hepatotoxicity, thrombotic microangiopathy (TMA), and other adverse and potentially lethal events. Administering an efficacious dose while managing potential immune responses requires gene-therapy developers to perform a delicate balancing act.
Multiple groups are engineering AAV capsids and transgene cassettes to improve transduction efficiency, with the goal of lowering vector doses. Promoters and enhancers can be modified for greater potency, and codon optimization can help to improve transgene expression. However, such approaches come with their own risks. Highly potent promoters and/or enhancers are associated with increased risk of oncogenesis in mouse models.
Transgenes can be engineered for greater activity. For example, hemophilia treatments based on a natural variant of human coagulation factor IX called the Padua variant have proven to be efficacious at comparatively low levels of transgene expression. However, engineered transgenes also can elicit immune responses, which could affect therapeutic efficacy and durability.
AAV capsids can be genetically modified for enhanced tropism, increased transduction efficiency, and reduced immunogenicity. One approach to achieving the latter goal is to introduce site-directed mutations to minimize antibody binding to capsid epitopes (13). Directed evolution of AAV capsids can be applied to select for viruses with the capacity to evade anti-AAV antibodies (14). Increased understanding of how the immune system responds to AAV has guided capsid engineering. As that knowledge grows, capsid designs will improve.
Immunosuppressive agents such as corticosteroids are other tools for preventing robust immune responses associated with high vector doses. Clinical trials have produced mixed results, with some studies showing enhanced transgene retention from coadministration of immunosuppressive agents and other studies showing no such effect. Studies in nonhuman primates (NHPs) suggest that timing of agent and AAV administration is critical (15). However, even if timing is tightly controlled, immunosuppressives expose patients to other potential complications, including increased risk of infections. As we learn more about the immunogenicity mechanisms of AAV-based therapies, broad immunosuppression is likely to be replaced by targeted approaches to modulate immune responses to the therapies themselves.
Targeted Immune Modulatory Agents
Use of targeted immunomodulatory agents alongside AAV gene therapies is promising, with multiple regimens in development. Coadministration could help to mitigate liver inflammation while enhancing AAV transduction and thus durability of treatment. Inhibiting T-cell responses and NAb formation also could enable vector redosing.
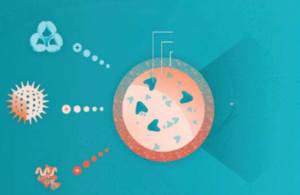
Figure 1: Coadministration of viral gene therapies — and other modalities — with immunomodualtory agents such as ImmTOR tolerogenic nanoparticles could improve therapeutic safety and efficacy. (PLA = polylactic acid, PEG = polyethylene glycol0l).
One such method is based on ImmTOR nanoparticles (Selecta Biosciences), which leverage rapamycin to generate antigen-specific immune tolerance (Figure 1). The nanoparticles are designed to accumulate in the spleen and liver following intravenous administration (16). There, they are taken up by antigen-presenting cells (APCs) such as dendritic cells (DCs), which are known to trigger immunogenic and tolerogenic responses from T cells. The particles promote induction of tolerogenic DCs, which go on to activate antigen-specific regulatory T cells. Preclinical studies have demonstrated the ability of ImmTOR technology to mitigate liver inflammation (17), enhance transgene expression (18), and enable vector redosing by inhibiting formation of anti-AAV antibodies (18, 19). In clinical studies, the nanoparticles mitigated formation of anti-AAV antibodies to a coadministered empty AAV capsid as many as 30 days after treatment (20). NHP studies have shown that three monthly doses of ImmTOR particles can inhibit NAb formation for at least 90 days (20).
Another approach is to administer immunoglobulin G (IgG) proteases for targeted removal of anti-AAV NAbs. This method has particular promise for the ~40% of patients who cannot receive AAV-based gene therapy because of preexisting antiviral NAbs. IgG proteases are bacterial enzymes that specifically cleave human IgG (21), potentially opening a treatment window for patients who otherwise would be ineligible (22, 23). One IgG protease in development, imlifidase, is derived from Streptococcus pyogenes, a common human pathogen. Most humans have been infected with S. pyogenes and have preexisting antibodies against it. Another example is the IdeXork IgG protease derived from a nonhuman pathogen. The enzyme cleaves human IgG specifically and efficiently and shows low cross-reactivity to human sera (24).
By combining approaches to mitigate immunogenicity, we are on the horizon of transforming the treatment paradigm of AAV-based gene therapies from “one and done” to “low and slow.” That shift could unlock the full potential of AAV gene therapies for patients in need.
References
1 Verdera HC, Kuranda K, Mingozzi F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Molec. Ther. 28(3) 2020: 723–746; https://doi.org/10.1016/j.ymthe.2019.12.010.
2 Mulligan RC. The Basic Science of Gene Therapy. Science 260(5110) 1993: 926–932; https://doi.org/10.1126/science.8493530.
3 Blacklow NR, Hoggan MD, Rowe WP. Isolation of Adenovirus-Associated Viruses from Man. Proc. Natl. Acad. Sci. USA 58(4) 1967: 1410–1415; https://doi.org/10.1073/pnas.58.4.1410.
4 Buchlis G, et al. Factor IX Expression in Skeletal Muscle of a Severe Hemophilia B Patient 10 Years After AAV-Mediated Gene Transfer. Blood 119(13) 2012: 3038–3041; https://doi.org/10.1182/blood-2011-09-382317.
5 Colella P, Ronzitti G, Mingozzi F. Emerging Issues in AAV-Mediated in Vivo Gene Therapy. Molec. Ther. Meth. Clin. Dev. 8, 2018: 87–104; http://dx.doi.org/10.1016/j.omtm.2017.11.007.
6 Pasi KJ, et al. Multiyear Follow-Up of AAV5-hFVIII-SQ Gene Therapy for Hemophilia A. New Engl. J. Med. 382(1) 2020: 29-40; https://doi.org/10.1056/NEJMoa1908490.
7 Li C, Samulski RJ. Engineering Adeno-Associated Virus Vectors for Gene Therapy. Nature Rev. Gen. 21(4) 2020: 255–272; https://doi.org/10.1038/s41576-019-0205-4.
8 Shirley JL, et al. Immune Responses to Viral Gene Therapy Vectors. Molec. Ther. 28(3) 2020 709–722; https://doi.org/10.1016/j.ymthe.2020.01.001.
9 Boutin S, et al. Prevalence of Serum IgG and Neutralizing Factors Against Adeno-Associated Virus Types 1, 2, 5, 6, 8, and 9 in the Healthy Population: Implications for Gene Therapy Using AAV Vectors. Hum. Gene Ther. 21(6) 2010: 704–712; https://doi.org/10.1089/hum.2009.182.
10 Ronzitti G, Gross DA, Mingozzi F. Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front. Immunol. 11, 2010: 670; https://doi.org/10.3389/fimmu.2020.00670.
11 Mingozzi F, High K. Immune Responses to AAV Vectors: Overcoming Barriers to Successful Gene Therapy. Blood 122(1) 2013: 23–36; https://doi.org/10.1182/blood-2013-01-306647.
12 Toxicity Risks of AAV Vector-Based Gene Therapy.
US Food and Drug Administration: Silver Spring, MD, accessed 2 September 2021; https://www.fda.gov/media/151950/download.
13 Tse LV, et al. Structure-Guided Evolution of Antigenically Distinct Adeno-Associated Virus Variants for Immune Evasion. Proc. Natl. Acad. Sci. USA 114(24) 2017: E4812–E4821; https://doi.org/10.1073/pnas.1704766114.
14 Ojala DS, et al. In Vivo Selection of a Computationally Designed SCHEMA AAV Library Yields a Novel Variant for Infection of Adult Neural Stem Cells in the SVZ. Mol. Ther. 26(1) 2019: 304–319; https://doi.org/10.1016/j.ymthe.2017.09.006.
15 Samelson-Jones BJ, et al. Timing of Intensive Immunosuppression Impacts Risk of Transgene Antibodies After AAV Gene Therapy in Nonhuman Primates. Molec. Ther. Meth. Clin. Dev. 17, 2020: 1129–1138; https://doi.org/10.1016/j.omtm.2020.05.001.
16 Kishimoto TK. Development of ImmTOR Tolerogenic Nanoparticles for the Mitigation of Anti-Drug Antibodies. Front. Immunol. 11, 2020: 969; https://doi.org/10.3389/fimmu.2020.00969.
17 Ilyinskii PO, et al. Enhancement of Liver-Directed Transgene Expression at Initial and Repeat Doses of AAV Vectors Admixed with ImmTOR Nanoparticles. Sci. Adv. 7(9) 2021: eabd0321; https://doi.org/10.1126/sciadv.abd0321.
18 Ilyinskii PO, et al. Enhancement of the Tolerogenic Phenotype in the Liver by ImmTOR Nanoparticles. Front. Immunol. 12, 2021: 637469; https://doi.org/10.3389/fimmu.2021.637469.
19 Meliani A, et al. Antigen-Selective Modulation of AAV Immunogenicity with Tolerogenic Rapamycin Nanoparticles Enables Successful Vector Re-Administration. Nature Comm. 9(1) 2018: 4098; https://doi.org/10.1038/s41467-018-06621-3.
20 Traber P, et al. Effect of Tolerogenic ImmTOR Nanoparticles on the Formation of Anti-AAV8 Antibodies in Mice, Non-Human Primates, and Healthy Human Volunteers. Annual Meeting of the American Society of Gene and Cell Therapy: Washington, DC, 16–19 May 2022 (poster presentation).
21 Jordan S, et al. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. New Engl. J. Med. 377(5) 2017: 442–453; https://doi.org/10.1056/NEJMoa1612567.
22 Leborgne C, et al. IgG-Cleaving Endopeptidase Enables in Vivo Gene Therapy in the Presence of Anti-AAV Neutralizing Antibodies. Nature Med. 26(7) 2020: 1096–1101; https://doi.org/10.1038/s41591-020-0911-7.
23 Ros-Gañán I, et al. Optimising the IgG-Degrading Enzyme Treatment Regimen for Enhanced Adeno-Associated Virus Transduction in the Presence of Neutralising Antibodies. Clin. Translat. Immunol. 11(2) 2022: 1375; https://doi.org/10.1002/cti2.1375.
24 Sjogren J, et al. Enzymatic Strategies for Eliminating Neutralizing Antibodies Prior to Administration of AAV Based Gene Therapies. Annual Meeting of the American Society of Gene and Cell Therapy: Washington, DC, 16–19 May 2022 (poster presentation).
Carsten Brunn is president and chief executive officer, and Kei Kishimoto is chief scientific officer at Selecta Biosciences, 65 Grove Street, Watertown, MA 02472; 1-617-923-1400; [email protected]; https://selectabio.com.
You May Also Like





