Gene Therapy Trends and Future ProspectsGene Therapy Trends and Future Prospects
May 24, 2020
Structure of adenoassociated virus, often used as a gene therapy vector (adapted from https://commons.wikimedia.org)
Gene therapy is defined as the transfer of genetic information to a patient for treatment of a disease. Clinical investigation of such therapies began in 1990 with a treatment for a rare immunodeficiency disorder and since has expanded to almost 1,000 clinical studies in 2019 (1, 2). In its most straightforward incarnation, the goal of gene therapy for genetic diseases is long-term expression of a transferred gene at levels that are high enough to be therapeutic, an approach sometimes called augmentation gene therapy. Transferred genes are most frequently normal copies of a mutated gene. Treatments also can suppress expression of detrimental genes through RNA interference or genome-editing tools. New editing technologies are opening up the possibility for correcting mutated genes in their precise genomic locations through homologous recombination with a donor template or use of base-editing technology (3). Although currently most approved gene-therapy products and products in late-stage clinical development are based on gene augmentation, some gene-editing strategies now are advancing into clinical testing and very well could change the commercial landscape in the near future.
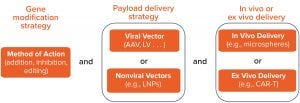
Figure 1: Gene therapy classification
Types of Gene Therapy
Figure 1 presents a framework for classifying the broad range of gene-therapy applications in modern development. The first level of classification relates to therapeutic intervention strategy — e.g., gene augmentation, suppression, or editing — and the specific molecular tools that are involved. The next layer of categorization relates to how a therapeutic drug substance will be delivered to cells, with two broad subcategories of viral and nonviral platforms.
Viral-based gene delivery exploits the evolutionary advantages developed by viruses to infect cells and then efficiently deliver their genetic payload for incorporation into those cells’ resident gene-expression machinery. The gene of interest is incorporated into a stripped-down version of a virus, which serves as a nonreplicating delivery vector that retains necessary viral elements to package and deliver a functional payload.
An important consideration is whether targeted cells will continue to divide (e.g., stem cells and T cells), in which case it is important that donated DNA will be incorporated into the host-cell genome for replication to be passed on to all daughter cells. For targeting long-lived postmitotic cells, which are no longer dividing, long-term expression of transferred DNA over each cell’s life span can be achieved through episomal stabilization (4).
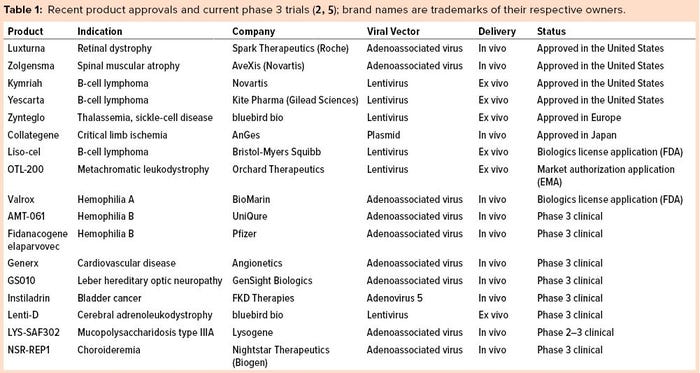
Several viral platforms have been developed over the years. But the field has coalesced around two types of viral vectors: lentiviral (LV) retrovirus vectors for genomic integration into dividing cells and adenoassociated viral vectors (AAVs) for gene transfer into postmitotic cells. Recent gene-therapy product approvals and products currently in phase 3 clinical studies are listed in Table 1, which highlights the use of these two viral vectors.
Note that the commercial and advanced clinical programs using LV vectors engineer patient cells ex vivo — qualifying both as autologous cell therapies and gene therapies. By contrast, products based on AAV vectors are administered in vivo as off-the-shelf allogeneic products. Although direct in vivo administration of an off-the-shelf product certainly would be preferred for all indications, many industry experts have been hesitant to administer LV vectors in vivo because of safety concerns relating to insertional mutagenesis as well as efficacy constraints due to rapid vector clearance and an inability to limit delivery exclusively to a targeted, desired cell type.
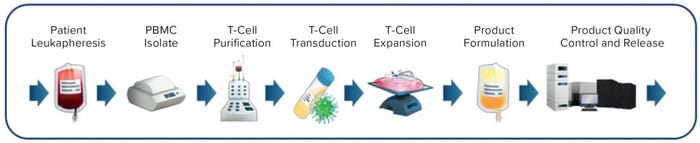
Figure 2: Ex vivo chimeric antigen receptor (CAR) T-cell manufacturing process (example adapted from www.chemometec.com)
Consequently, the desired cells are isolated from a patient, then genetically modified ex vivo for returning to the same patient (Figure 2). The ex vivo process introduces significant manufacturing and sample-shipping complexities, requires patient-specific (autologous) product batches to prevent cell rejection on subsequent readministration, creates an unprecedented cost of goods (CoG), and thus is not considered to be a sustainable practice for the long-term future. Second-generation approaches using allogeneic rather than autologous cell sources are in development now to eliminate the requirement for individual patient batches. However, costs and challenges associated with ex-vivo cell engineering and expansion remain an issue. Some efforts also are under way to develop truly off-the-shelf LV-based gene therapies for direct in vivo administration (6, 7).
Nonviral gene-delivery methods could address limitations of viral-based delivery and offer the following potential advantages:
ease of chemical characterization
simplicity and reproducibility of production
capacity for larger cargo packaging
reduced biosafety concerns.
However, such gene-delivery methods are relatively inefficient compared with recombinant viral systems, necessitating large therapeutic doses that bring associated toxicity concern. So far, that has limited the use of nonviral technologies severely. Nonetheless, improvements to nucleic-acid stability (and consequently, potency) as well as lipid and polymer delivery technologies are advancing the field (8). Recently, Smith et al. used nanoparticle-based delivery to demonstrate an in vivo chimeric antigen receptor (CAR) T-cell application in a mouse model (9). The researchers achieved persistent CAR expression in actively dividing T cells using a transposon gene-editing strategy for integrating the transgene into a host-cell genome with similar results to those achieved using LV vectors.
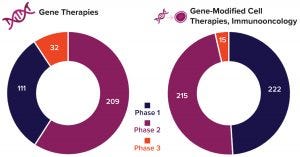
Figure 3: Number of gene-therapy clinical trials in progress by the end of 2019 (2)
Gene Therapy Prospects
Given the increasing number of clinical studies that are under way and tremendous mergers, acquisitions, and venture-capital investments in the cell and gene-therapy business overall, the future indeed looks bright for gene therapies. Figure 3 illustrates the product pipeline as of the start of 2020 according to the Alliance for Regenerative Medicine, and Figure 4 compares related finances for the past three years (2). However, considerable technical challenges remain to be solved, with some of the most important requirements briefly introduced below.
Figure 4: Global regenerative-medicine financing by type (2017–2019) (2); US$7.6 billion total financing for gene and gene-modified cell therapies made 2019 the second-highest value year on record for the industry.
Increase Supplies of Viral Vectors: Expanding the available supply of viral vectors starts with good manufacturing practice (GMP)–produced plasmids and will require significant advances in viral-vector manufacturing to both increase batch size and decrease manufacturing costs. Another opportunity to address supply constraints and high cost could come through increasing vector potency and thereby decreasing therapeutic dose requirements.
Improve Purity of AAV Preparations: Current cell-culture methods for producing AAVs from triple transfection with plasmids could produce batches in which empty capsids are more abundant than those with the desired genetic cargo (10).
Improve Ex Vivo Cell-Based Processing Logistics: Near-term opportunities include adoption of automated, closed production processes and moving to allogeneic cell sources. A longer-term opportunity might be to obviate the need for ex vivo genetic modification of cells through direct in vivo delivery to intended cell targets, which would require development of safe and appropriately cell-specific products.
Overcome Preexisting Immunity to Viral Vectors: Patient immunity is a significant issue for AAV-based gene therapies because a significant portion of the population has preexisting antibodies to AAV (11). That precludes such treatment or limits it to a single administration through development of neutralizing antibodies. Solutions might include development of modified AAV strains, development of new vectors based on new viruses, and improvement of nonviral delivery methods.
Increase Therapeutic Durability: Improving the systemic half-life of gene therapies will require eliminating and/or minimizing immune responses (both innate and adaptive) to them.
References
1 Anderson WF. September 14, 1990: The Beginning. Hum. Gene Ther. 1, 1990: 371–372; https://doi.org/10.1089/hum.1990.1.4-371.
2 Advancing Gene, Cell, and Tissue-Based Therapies: ARM Annual Report and Sector Year in Review — 2019. Alliance for Regenerative Medicine: Washington, DC, 2019; https://alliancerm.org/publication/2019-annual-report.
3 Komor AC, et al. Programmable Editing of a Target Base in Genomic DNA Without Double-Stranded DNA Cleavage. Nature 533, 2016: 420–424; https://doi.org/10.1038/nature17946.
4 Lufino MMP, Edser PAH, Wade-Martins R. Advances in High-Capacity Extrachromosomal Vector Technology: Episomal Maintenance, Vector Delivery, and Transgene Expression. Mol. Ther. 16(9) 2008: 1525–1538; https://doi.org/10.1038/mt.2008.156.
5 Philippidis A. 25 Up-and-Coming Gene Therapies of 2019. Gen. Eng. Biotechnol. News 20 May 2019; https://www.genengnews.com/a-lists/25-up-and-coming-gene-therapies-of-2019.
6 Milani M, et al. Phagocytosis-Shielded Lentiviral Vectors Improve Liver Gene Therapy in Nonhuman Primates. Sci. Transl. Med. 11(493) 2019: 1–13; https://doi.org/10.1126/scitranslmed.aav7325.
7 Palfi S, et al. Long-Term Follow-Up of a Phase I/II Study of ProSavin, A Lentiviral Vector Gene Therapy for Parkinson’s Disease. Hum. Gene Ther. Clin. Dev. 29(3) 2018: 148–155; https://doi.org/10.1089/humc.2018.081.
8 Cullis PR, Hope MJ. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 25, 2017: 1467–1475; https://doi.org/10.1016/j.ymthe.2017.03.013.
9 Smith TT, et al. In Situ Programing of Leukaemia-Specific T Cells Using Synthetic DNA Nanocarriers. Nat. Nanotech. 12(8) 2017: 813–820; https://doi.org/10.1038/nnano.2017.57.
10 Schnödt M, Büning H. Improving the Quality of AAV Vector Preparations: The Challenge of Product-Related Impurities. Hum. Gene Ther. Meth. 28(3) 2017: 101–108; https://doi.org/10.1089/hgtb.2016.188.
11 Stanford S, et al. Adenovirus-Associated Antibodies in UK Cohort of Hemophilia Patients: A Seroprevalence Study of the Presence of Adenovirus-Associated Virus Vector-Serotypes AAV5 and AAV8 Neutralizing Activity and Antibodies in Patients with Hemophelia A. Res. Pract. Thromb. Haemost. 3, 2019: 261–267; https://doi.org/10.1002/rth2.12177.
Neal F. Gordon, PhD, is managing director of BioProcess Technology Group BDO USA, LLC, One International Place, Boston, MA 02110; 1-781-307-1426; [email protected]; https://www.bdo.com/industries/life-sciences/bioprocess-technology.
You May Also Like






