Meeting the Challenges in Manufacturing Autologous Cellular TherapiesMeeting the Challenges in Manufacturing Autologous Cellular Therapies
March 1, 2011
Personalized medicine is a promising new approach to disease treatment. The ultimate in personalized medicine is a cellular therapy manufactured specifically for an individual patient using his or her own cells. But this autologous approach to generating immunotherapies has unique manufacturing challenges. Each patient receives an individual product batch, which needs to be manufactured, tested, and released. So thousands to tens of thousands of batches could be made for each indication every year. Given the personalized nature of these therapies, the production scale remains the same for each batch. Thus, scale-up is not required; scale-out is key for meeting the demands of autologous cell-therapy manufacturing.
Manufacturing Methods
Generating autologous cellular therapies can take several approaches. In general, all involve obtaining autologous cells from a patient by procedures performed at a hospital or blood center. Depending on the therapy, those cells are either transferred to a local laboratory for further processing or shipped to a central processing facility. This starting material has limited stability, requiring immediate processing upon receipt. For proliferating cells, the desired cell type for a given therapy is typically expanded in cell culture. If the cells are terminally differentiated (nonproliferating), then cells for the therapy are typically generated by isolating precursor cells for the desired type and culturing them with appropriate cytokines for differentiation into the desired cell type. During cellular processing, an antigen may be added to elicit a desired immune response. This may be a defined antigen (the same for every patient and process) that can be produced using traditional large-scale methods. For example, Dendreon Corporation uses a defined-antigen approach in making its Provenge (sipuleucel-T) prostate cancer treatment, which received the first and only FDA approval to date for an autologous cellular therapy in April 2010. The antigen for Provenge is a recombinant fusion protein comprising granulocyte macrophage–colony stimulating factor (GM-CSF) crosslinked to the prostate antigen prostatic acid phosphotase (PAP). This recombinant fusion protein (PAP-GM-CSF) is manufactured at large scale to provide material for multiple batches of product. Other manufacturing approaches use an autologous antigen from each patient’s tumor for oncology treatment or from a given virus for treating infectious diseases. They generate completely autologous cellular therapies tailored to each individual’s disease. This enables adaptation of methods from one indication to another without the need to identify defined antigens for each indication. However, it also increases the complexity of the manufacturing process because an antigen needs to be processed and tested for each patient and combined with that patient’s own cells.
Photo 1:

There are different methods for processing autologous antigens. The simplest method is to use tumor lysate, which requires relatively minimal processing, essentially a homogenization step. That provides a patient’s cells directly with the proteins expressed by his or her tumor as antigens during cellular processing to generate the immunotherapy. Alternatively, tumor lysate, can be processed to isolate total RNA, enabling cells of interest to express and process it for the immunotherapy.
This can be taken a step further by amplifying the mRNA in the isolated total RNA. Although that requires the most effort in terms of antigen processing, a relatively small amount of tumor is required to generate sufficient mRNA for multiple doses (or even multiple batches) of the cellular therapy. Following cellular processing, the resulting cellular therapy may be either directly infused (so one manufacturing run generates one dose for the patient) or cryogenically preserved (in multiple doses).
Once cryogenically preserved, each individual dose for a patient is then delivered using liquid nitrogen dry shippers before administration. In early phase clinical trials, the manufacturing of cellular therapies generally uses manual processing methods transferred from research laboratories. Semiautomated commercial equipment options are available for the isolating precursor cells. Some instruments use antibody-coated bead–based methods (e.g., the CliniMACS cell separation system from Miltenyi Biotec, www.miltenyibiotec.com) for isolating precursor cells. Another is based on elutriation, also known as counterflow centrifugation (the Elutra cell separation system from CaridianBCT Inc., www.caridianbct.com).
Some companies have developed their own proprietary methods for isolating precursor cells. Examples include a tangential-flow filtration method developed by Northwest Biotherapeutics, Inc. (www.nwbio.com) and a cell separation device used by Dendreon. Options abound for the initial isolation of precursor cells. But little automated commercial equipment is available to address either the remaining cellular processing steps or the more complex antigen processing steps.
Some companies are working to develop cellular therapy enabling devices, such as Miltenyi’s CliniMACS Prodigy system. But a need remains for automated equipment that can handle the complexity of autologous therapy processing and enable scale-out for larger clinical trials and commercialization. Therefore, Argos Therapeutics initiated development of its own automated system to address these manufacturing challenges.
The key to scale-out for autologous cellular therapies is using functionally closed, single-use technologies and automation for processing. Such disposables minimize cleaning requirements between batches — which is critical for turnover of equipment — and eliminate concerns regarding cross contamination. Automation provides consistency and efficiency in processing using disposables.
A Custom Design Argos’s Arcelis platform generates monocyte-derived dendritic-cell products in clinical development for oncology and infectious disease. The technology uses amplified RNA from a patient’s tumor sample as an antigen (Figure 1). In collaboration with Invetech Pty. Ltd. (www.invetech.com.au), Argos is developing automated equipment that uses functionally closed disposables to enable commercial manufacturing.
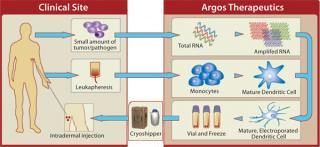
White blood cells obtained from patients through leukapheresis are shipped to the Argos central manufacturing facility for processing. Isolation of monocytes (the precursor cells used to generate dendritic cells) is performed using a functionally closed disposable and the existing semiautomated Elutra cell separation system. Two additional devices are in development to perform the remaining cellular processing steps: media-exchange steps required to prepare the cells for culture, electroporation (the process used to introduce antigen RNA into cells), and formulation and fill of the drug product.
Because the drug product formulation uses each patient’s own plasma as an excipient, one device processes the plasma, which is collected during leukapheresis, and prepares it for use in the final formulation of the cellular therapy. Patient material never comes into contact with the equipment, only the disposable, which prevents cross contamination and minimizes cleaning between processes.
The use of tube sealers and tube welders enables movement (removal or addition) of bags or reagents as required. For example, when culture bags are filled with the appropriate cells, media, and cytokines, they are removed using a tube sealer to be incubated for the required five days. When cells need harvesting, those culture bags are attached to the appropriate disposable using a tube welder. Methods are in place to verify that the appropriate patient’s cells will be welded onto the disposable. Similar methods verify that the correct tubes are being welded before welding is enabled for all reagents and in-processing materials.
Argos and Invetech designed a third prototype device (Photo 1) to process tumor homogenates to isolate and amplify RNA inside a functionally closed disposable container. This single-use technology has been uniquely designed to ensure that equipment does not come into contact with patient material (Figure 2). Again, this eliminates concerns for cross contamination between processes. It also eliminates the need for cleaning validation, which would be extremely challenging because the product generated during this process is amplified nucleic acid.

The disposable containment consists of a rigid tray incorporating a PCR plate for nucleic acid amplification. It also contains washing and elution stations for isolation and purification steps. A disposable pump head is incorporated to generate a closed-loop vacuum for isolation and purification using silica columns. An incorporated reagent rack can be cooled to ensure reagent stability. All necessary reagents are foil-sealed until required for processing. There are tip racks to hold new and used pipette tips for all liquid transfers. A flexible barrier top with an incorporated pipette head is sealed onto the rigid tray. The barrier allows a pipette head to access all areas inside the tray: tip racks, reagent racks, PCR plate, and washing/elution stations.
The device contains analytical equipment needed to determine concentration of the nucleic acid, with spectrophotometric cuvettes incorporated in the rigid tray for such testing. Volumetric cuvettes are designed to enable determination of the volume of resulting nucleic acid solutions generated to calculate yields and volumes required for concentration normalization.
The thermal cycler lid moves through the side of the disposable through a rolling boot, enabling it to move in and out to cover the PCR plate during incubations and allowing pipette access for transfers to and from the plate. Overall, this equipment and disposable design provides a fully automated method for RNA isolation and amplification from tumor homogenate in a single-use format. This is critical for meeting the demands of commercial manufacturing for Argos Therapeutics.
More Challenges
In addition to the need for automated processing equipment, other critical considerations come up when processing autologous cellular therapies on a commercial scale. The need to process the patient’s cellular samples immediately upon receipt of them makes knowing when a therapy is prescribed and scheduling of their collection critical. If an antigen is autologous as well, then companies must ensure that material is received, processed, and ready to be incorporated into cellular processing.
Given the unique traceability requirements for autologous cellular therapies, mechanisms to ensure that multiple patient product streams are combined correctly and that the correct product is delivered to each patient are essential. The extraordinarily high number of batches being tested and released for commercialization needs to be addressed. The complexity of process scheduling, traceability, and release issues for autologous cellular therapies requires a well-designed manufacturing execution system to manage those activities and enable electronic batch records with release by exception. For the promise of personalized cellular therapies to be fully realized, effective methods need to be developed that will address all these challenges and complexities of their manufacture.
About the Author
Tamara T. Monesmith is director of manufacturing and process development at Argos Therapeutics, 4233 Technology Drive, Durham, NC 27704; 1-919-287-6321, fax 1-919-287-6301; [email protected], www.argostherapeutics.com.
REFERENCES
1.) Goldman, B, and L. DeFrancesco. 2009. The Cancer Vaccine Roller Coaster. Nat. Biotechnol. 27.
2.) Nicolette, C. 2007. Dendritic Cells for Active Immunotherapy: Optimizing Design and Manufacture in Order to Develop Commercially and Clinically Viable Products. Vaccine 25:B48-B60.
3.) Whiteside, TL. 2008. Evaluation of Dendritic Cell Products Generated for Human Therapy and Post-Treatment Immune Monitoring. BioPharm Int. 21:42-67.
4.) Miesowicz, f. 2007. Dendritic-Cell Based Therapies. Genetic Eng. News 27:44-46.
5.) Finke, LH. 2007. Lessons from Randomized Phase III Studies with Active Cancer Immunotherapies: Outcomes from the 2006 Meeting of the Cancer Vaccine Consortium (CVC). Vaccine 25S:B97-B109.
6.) Osada, T. 2006. Dendritic Cell-Based Immunotherapy. Int. Rev. Immunol. 25:377-413.
7.) Hoos, A. 2007. A Clinical Development Paradigm for Cancer Vaccines and Related Biologics. J. Immunother. 30:1-15.
8.) Kantoff, PW. 2010. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 363:411-422.
9.) Fitzpatrick, I. 2008. Cellular Therapy Success Through Integrated Automation. BioProcess Int. 6:S32-S37.
10.) Rios, M. 2010. Flexible Manufacturing. BioProcess Int. 8:34-46.
You May Also Like






