Microfluidic Cell Sorting: Scaling for Clinical and Therapeutic ApplicationsMicrofluidic Cell Sorting: Scaling for Clinical and Therapeutic Applications
Regenerative medicine is ushering in a paradigm shift focused on therapeutic cell delivery for personalized medicine. Mesenchymal stromal cells (MSCs) have garnered extensive attention from both academic and industrial sectors since their discovery over 50 years ago. The unique properties of MSCs, including their multilineage differentiation and immunomodulatory effects, have propelled research into the pursuit of therapeutic applications. To date, 474 clinical trials have been completed using MSCs to treat an array of diseases, such as acute myocardial infarction, knee osteoarthritis, rheumatoid arthritis, and diabetes (1–4).
Despite the widespread interest in MSCs, they are scarce in human bodies, constituting just 0.01% and 0.005% of the bone-marrow mononucleated cell (BMMNC) population for males and females, respectively (5). With such low cell counts, large volumes of bone-marrow aspirate (BMA) and extended culture times are needed for sufficient MSC expansion in downstream processing and therapeutic applications. This situation underscores the pressing need for increased efficiency in methods used for extracting MSCs from biological samples.
Challenges with Traditional Extraction Methods
Based on antibody labeling of cell-surface markers, fluorescence-activated cell sorting (FACS) is widely regarded as the gold standard for cell separations. However, the antibody labels render sorted cells unsuitable for therapeutic use because of potential effect on native biological signaling pathways.
Another commonly used method for cell separation/enrichment is Ficoll density-gradient centrifugation (DGC), which leverages the differing densities of cellular components present in samples. For example, erythrocytes and polymorphonuclear (PMN) cells can be separated from mononuclear cells (MNCs) through Ficoll density-gradient medium from Cytiva using centrifugal forces. During this type of separation from BMA (a critical first step in MSC-therapeutic manufacturing), MSCs are assumed to reside in the mononuclear population. However, they also can aggregate to form clusters of cells, causing them to segregate into the PMN population, which often gets discarded after processing (6).
In addition to the resulting low MSC yields, the Ficoll DGC technique is limited by susceptibility to inherent variability due to heavy reliance on manual processing. Given the importance of cell fractionation in biological research and cell-therapy manufacturing, commercial products such as Sepax (Cytiva) cell processing and CTS Rotea counterflow centrifugation (Thermo Fisher Scientific) have been made available on the market. Both options can process different biological samples such as diluted whole blood, apheresis products, and tissue sources (e.g., BMA, cord blood, and adipose tissues), albeit at high instrument and consumables costs but limited scalability. Still, MSC yields by centrifugation from BMA can be as low as 13% (7).
Microfluidic Technologies
Recent advances in microfluidics have enabled miniaturization and automation of high-precision cell sorting. Use of microfluidic cell-sorting techniques provides high-purity cell recoveries with little variability and at a relatively low cost. However, scaling such technology to process large volumes (necessary for manufacturing applications) remains difficult. Because microfluidics technologies sort cells according to their intrinsic properties rather than labeling them, translating the technology for higher throughput is worth the effort.
Microfluidic sorting of MSCs has been demonstrated with the inertial focusing technique (8–10). The emphasis of those studies, however, was to sort out subpopulations of particular sizes from heterogeneous MSC cultures. Translating the technology to process undiluted samples will not work well because of its low sample-concentration requirement for sorting.
As an alternative, the deterministic lateral displacement (DLD) sorting technique has demonstrated proficiency in processing extremely concentrated samples with promising cell recoveries, making it more suitable for clinical applications. This technique operates on a fundamental principle of fluid mechanics around micropillar arrays. The strategic arrangement of those arrays allows particles that are larger than a cut-off specification (critical diameter) to follow a unique, deterministic trajectory away from their original flow path. By contrast, particles that are smaller than the set specification will not be perturbed and thus continue their original fluid-flow trajectory. This provides for a clear separation between small and large particles.
The Critical Analytics for Manufacturing Personalized-Medicine (CAMP) interdisciplinary research group (IRG) is part of the Singapore-MIT Alliance for Research and Technology (SMART), which is the Massachusetts Institute of Technology’s research enterprise in Singapore. The CAMP-IRG has developed an innovative stem-cell sorting platform that addresses the challenges of large-scale cell therapy manufacturing using DLD microfluidic sorting technology. Our platform can process small bone-marrow samples (2.5 mL) in 20 minutes, providing double the stem-cell yield of established methods without costly reagents or complex processes (11).
To demonstrate the utility and proficiency of DLD sorting for clinical applications, we performed a series of experiments to validate and benchmark the technology against a conventional MSC isolation method (Ficoll DGC) using a clinical BMA sample, the most abundant source of MSCs. To overcome the previously low throughput of DLD (due to high fluidic resistance from the micropillar arrays), we devised a high-throughput sorting method by parallelizing four DLD sorting devices into a single microfluidic chip (25 mm × 75 mm). To complement that, we also built a multiscale microfluidics platform intended to operate up to five chips for increasing the processing throughput further (Figure 1).
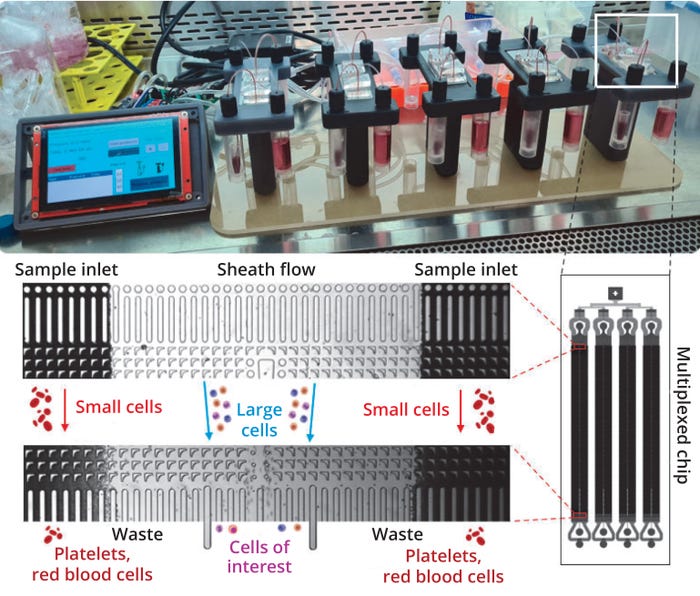
Figure 1: A high-throughput design integrates four deterministic lateral displacement (DLD) sorting arrays in parallel on a single chip to increase sorting speed. Throughput is increased further by a custom sorting system that enables up to five chips to operate simultaneously.
Experimental Validation
First, we demonstrated size-based sorting efficiency using a mixture of beads (4.4 µm and 7.3 µm) in our DLD sorting device, with a critical diameter set at 5.6 µm. The sorting efficiency remained high (>95%) across different flow conditions, ensuring consistent sorting at varying flowrates.
Next, we used the high-throughput chip on our multiscaled platform for clinical bone-marrow samples. We filtered those using a 20-µm filter to remove unwanted cell coagulation before sorting. Apart from that filtration, however, we sought to preserve the physiological relevance of the MSCs by performing no additional preprocessing steps (e.g., dilution). To benchmark our sorting performance, we also ran a Ficoll DGC process in tandem. Results from comparing both sorting methods showed that DLD sorting enriched MSCs twice as efficiently (43.9 ± 6.3% compared with 16.6 ± 6.3% for Ficoll DGC), with a 10-fold reduction in sorting time and minimal difference in MSC markers after both sorting methods (Figure 2).
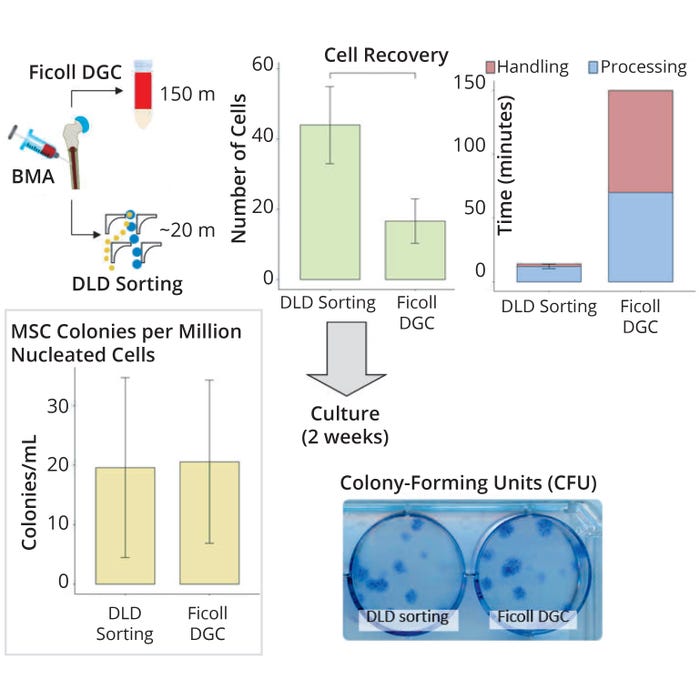
Figure 2: Sorting efficiency and recovery of mesenchymal stem cells (MSCs) from bonemarrow aspirates (BMAs) using a deterministic lateral displacement (DLD) device and Ficoll density-gradient centrifugation (DGC) processing to validate the effectiveness of MSC enrichment based on colony-forming unit (CFU) count, cell recovery, and time (n = 3).
Outlook
Our study shows that DLD provides superior sorting efficiency and effectiveness over Ficoll DGC cell separations. These results suggest that fewer samples could be extracted to achieve an equivalent number of cells, reducing discomfort for patients/donors in cell manufacturing and transplant applications. In the context of autologous MSC cell therapy, patients often begin treatment with an illness, which can make it difficult to obtain necessary aspirate volumes (often ~50 mL) (12). That predicament can render some patients ineligible for therapy or necessitate increased cell passages to meet manufacturers’ optimal MSC dose requirements, thus extending processing time and delaying therapeutic delivery. It is known that the “stemness” of MSCs also decreases with increased passages (13). Therefore, it is important to extract as many MSCs as possible for processing. Note that eliminating the requirement for sample preparation can reduce sample-to-product time as well as contamination risk, facilitating the future development of this technology.
We plan to improve the sorting efficiency and throughput of our technique so that future aspirations will require a lower starting volumes to obtain the same or higher yields as available with current standards — but with reduced processing time. That will be achieved through device parallelization, chip stacking, and changing device parameters for higher sorting specifications.
The functional qualities and immunomodulatory properties of the resulting MSCs also will need testing through differentiation and functional assays. Although such tests were not part of our recent study, it is important to recognize that the potential application of DLD sorting extends beyond just sample processing. The method could streamline processes by eliminating laborious steps such as dilution and multiple-step protocols.
Our current efforts focus on exploring alternative microchip-fabrication technologies for rapid manufacturing with high turnaround times. Such an alternative could enable us to scale production for industrial demands and reduce costs with enhanced mechanical properties. Concurrently, we are developing a second version of the sorting technology that houses eight sorting devices in an improved design for achieving higher cell recovery.
In the long term, we hope to broaden the scope of this technology beyond BMA processing into applications such as apheresis. As the biopharmaceutical industry transitions into a new era focused on personalized medicine for medical treatment, incorporating such sorting technologies offers promise for augmenting biomanufacturing processes overall.
References
1 Carbone RG, et al. Stem Cells Therapy in Acute Myocardial Infarction: A New Era? Clin. Exp. Med. 21, 2021: 231–237; https://doi.org:10.1007/s10238-021-00682-3.
2 Weiss JN. Chapter 27: Allogeneic Adipose Tissue-Derived Mesenchymal Progenitor Cells for the Treatment of Knee Osteoarthritis. Orthopedic Stem Cell Surgery. Weiss JN, Ed. Springer Nature Switzerland: Charn, Switzerland, 2021; 149–153.
3 Sarsenova M, et al. Mesenchymal Stem Cell-Based Therapy for Rheumatoid Arthritis. Int. J. Mol. Sci. 22(21) 2021: https://doi.org:10.3390/ijms222111592.
4 Weiss JN. Chapter 29: Allogeneic Mesenchymal Human Stem Cell Infusion Therapy for Endothelial Dysfunction in Diabetic Subjects (ACESCO). Stem Cell Surgery Trials in Heart Failure and Diabetes: A Concise Guide. Weiss JN, Ed. Springer Nature Switzerland: Charn, Switzerland, 2022: 151–155.
5 Li J, et al. Factors Affecting Mesenchymal Stromal Cells Yield from Bone Marrow Aspiration. Chin. J. Cancer Res. 23, 2011: 43–48; https://doi.org:10.1007/s11670-011-0043-1.
6 Ahmadbeigi N, et al. The Aggregate Nature of Human Mesenchymal Stromal Cells in Native Bone Marrow. Cytother. 14(8) 2012: 917–924; https://doi.org:10.3109/14653249.2012.689426.
7 Mareschi K, et al. Multipotent Mesenchymal Stromal Stem Cell Expansion By Plating Whole Bone Marrow at a Low Cellular Density: A More Advantageous Method for Clinical Use. Stem Cells Int. 2012: 920581; https://doi.org/10.1155/2012/920581.
8 Yin L, et al. Label-Free Separation of Mesenchymal Stem Cell Subpopulations with Distinct Differentiation Potencies and Paracrine Effects. Biomaterials 240, 2020: 119881; https://doi.org:https://doi.org/10.1016/j.biomaterials.2020.119881.
9 Yin L, et al. Microfluidic Label-Free Selection of Mesenchymal Stem Cell Subpopulation During Culture Expansion Extends the Chondrogenic Potential In Vitro. Lab Chip 18(6) 2018: 878–889; https://doi.org/10.1039/c7lc01005b.
10 Lee WC, et al. Multivariate Biophysical Markers Predictive of Mesenchymal Stromal Cell Multipotency. Proc. Nat. Acad. Sci. 111(42) 2014: E4409–E4418; https://doi.org/10.1073/pnas.1402306111.
11 Zen NTK, et al. Scalable Mesenchymal Stem Cell Enrichment from Bone Marrow Aspirate Using Deterministic Lateral Displacement (DLD) Microfluidic Sorting. Lab Chip 23(19) 2023: 4313–4323; https://doi.org/10.1039/D3LC00379E.
12 Gudleviciene Z, et al. Quick and Effective Method of Bone Marrow Mesenchymal Stem Cell Extraction. Open Med. 10(1) 2015: 44–49; https://doi.org/10.1515/med-2015-0008.
13 Yang Y-HK, et al. Changes in Phenotype and Differentiation Potential of Human Mesenchymal Stem Cells Aging In Vitro. Stem Cell Res. Ther. 9(131) 2018; https://doi.org/10.1186/s13287-018-0876-3.
Nicholas Tan Kwan Zen is a research engineer, corresponding author Jongyoon Han is co-lead principal investigator, and Kerwin Kwek Zeming is a senior research scientist, all in the Critical Analytics for Manufacturing Personalized-Medicine (CAMP) interdisciplinary research group at the Singapore-MIT Alliance for Research and Technology (SMART) in Singapore; [email protected]; https://smart.mit.edu.
You May Also Like





