Platform Solutions for Cell Therapy ManufacturingPlatform Solutions for Cell Therapy Manufacturing

Figure 1: Efficiency of Lonza bioreactor platform over traditional 2D planar culture methods
Advances in cell therapy have resulted in significant progress toward treating some widespread and difficult diseases, many of which represent unmet medical needs. For example, phase 3 clinical trials are already under way for therapies based on mesenchymal stem cells (MSCs), including therapies for graft-versus-host disease, acute myocardial ischemia, and chronic obstructive pulmonary disease (COPD) (1–3).
Successful cell therapy treatments for such afflictions will be not only significant medical breakthroughs, but also in very high demand. However, their commercialization is currently limited by high cost of goods (CoGs) and manufacturers’ inability to scale up manufacturing while maintaining their products’ critical quality attributes. The objective is to develop and integrate manufacturing approaches that will address those gaps.
View the full article below – Login Required
Platform Considerations
The ideal cell therapy platform should be scalable and robust. It should maximize final product yield while keeping costs low and a final product quality at a high standard. For example, Lonza’s single-use bioreactor platform has achieved approximately 80-fold more cells per given volume than with traditional two-dimensional (2D) planar methods (Figure 1) and hence the reduced costs for an affordable cell therapy solution. This improved efficiency is the result of using the right combination of cell culture technologies, including microcarriers, single-use vessels, real-time process parameter monitoring and control tools, and overall bioreactor architecture with optimized procedures related to cell inoculation, cell expansion, feeding strategies, and harvesting methods.
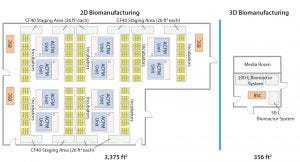
Figure 2: Comparison of 2D and 3D manufacturing footprint for the production of 1 trillion mesenchymal stem cells (MSCs)
With this increased efficiency in cell expansion, the demands and costs associated with maintaining commercial good manufacturing practices (GMP) suite space decreases. For example, in a process modeled for manufacturing one trillion cells for a stem cell therapy, the required GMP manufacturing space can be reduced by almost 10-fold using a bioreactor platform in lieu of traditional 2D planar cell culture systems (Figure 2). Furthermore, the reduced space and equipment requirements can substantially reduce costs associated with labor, facilities upkeep, cleaning, and validation of the space between processes.
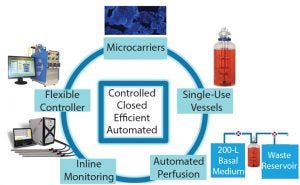
Figure 3: Components of Lonza’s cell therapy bioreactor platform for adherent cells
To achieve the optimal bioreactor platform for a wide range of processes, considerations regarding growth of cells, vessel capacity limitations, vessel materials (e.g., stainless steel, glass, or single-use), feeding strategies, agitation profiles, process monitoring, and appropriate control and feedback loops should be evaluated to ensure process robustness, reproducibility, and cost effectiveness. Overall, technologies chosen for any expansion process should be controlled, efficient, automated, and used a closed system to decrease risk of contamination.
Microcarrier Selection
For anchorage-dependent cells, microcarriers provide a larger ratio of surface area to volume than do traditional 2D culture vessels, typically resulting in higher cell yields. A number of commercially available microcarriers are available for anchorage-dependent cell expansion in 3D bioreactor systems. Manufacturers of such technologies offer a range of coatings, materials, colors, and florescence to fit individual process needs. Common drawbacks of using microcarriers include the tendency of such cultures to form large aggregates and the necessity to dissociate them during cell harvest. And cells harvested from microcarriers typically require an additional downstream process to separate microcarriers from a cell product. However, those challenges often are overcome in the optimization phase of process development.
Microcarriers can vary in their surface topography, each having its own advantages and challenges. Three standard microcarrier surface types are smooth, microporous, and macroporous. Smooth microcarriers are best used in culture environments where cells are not adversely affected by fluid sheer. Cells are typically removed easily from such surfaces and are less likely to create heterogeneous microenvironments. Microporous microcarriers have pores that are too small for cells to infiltrate, yet they are capable of allowing various nutrients to diffuse across the microcarriers’ inner structures.
Macroporous microcarriers have pore sizes ranging 30–400 µM (4), providing maximum surface area in a three-dimensional (3D) structure to enhance cell anchorage to the surface. Some cell types also can grow within the larger pores, providing protection from sheer forces created by agitation and sparging. Macroporous microcarriers allow cells to grow within them as well as on the carrier surface, which can create heterogeneous microenvironments. When using macroporous microcarriers, it is very important to evaluate cell characteristics postharvest to ensure that microenvironment formation does not cause significant changes in a final cell product.
Culture Vessel
Whether for suspension or anchorage-dependent cell expansion, selection of an appropriate culture vessel is crucial. Different vessel options are available, each with unique benefits and challenges. Key distinctions between bioreactor vessels are build material, working/total volume, agitation technologies, integrated sensor capabilities, scalability, and automation.
One notable trend in biomanufacturing over the past few years has been the use of single-use vessels in place of traditional stainless steel or glass culturing vessels because of their ease of use and cost effectiveness. Spinner flasks are very inexpensive, easy-to-use, single-use cell expansion vessels for initial 3D studies and are available in various working volumes. However, spinners lack the culture monitoring and control functions of bioreactors. As a result, some vendors now offer small-scale bioreactor systems ranging from 10-mL to 1-L working volumes as a more advanced option for small-scale research applications. Those systems can automate complex design of experiments (DoE) protocols for high-throughput screening and online, real-time monitoring of a culture.
For commercial- or clinical-stage projects, single-use bioreactor systems are available with working volumes ranging from 3 L to >1,000 L. Many of these platforms are equipped with disposable pH and dissolved oxygen (DO) sensors, but they can accommodate auxiliary sensors, stainless steel sensor probes, or automated sampling apparatuses through integrated vessel ports. Although it may be beneficial to explore such new technologies at the early stages of a project, it is also extremely important to consider how, or whether, each chosen technology will scale-up to achieve adequate commercial production lot sizes.
In addition, different media agitation technologies are available beyond traditional stirred-tank impellers. Bioreactor vessels now use shaking platforms, static culture chambers with recirculating media exchange, rocker platforms, and paddle-wheel designs to provide adequate gas exchange and nutrient availability to the culture system. Many of those vessels also are available as single-use designs. However, initial capital costs associated with use of disposable vessels and the controller system for the bioreactor should be considered when seeking the appropriate, cost-effective platform. Also, the biocompatibility of such disposable vessels should be thoroughly evaluated to prevent detrimental effects on the manufacture of a cell therapy product.
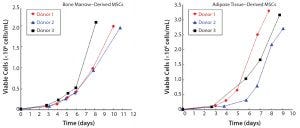
Figure 4: Bioreactor expansion of human MSCs in 2-L stirred-tank bioreactor; viability >95%
Cell Expansion Example
We cultured human-bone marrow–derived mesenchymal stem cells (hBM-MSCs) and human adipose tissue-derived MSCs (hAT-MSCs) using Lonza’s proprietary platform and protocols. We produced over 2 × 106 hBM-MSCs cells/mL and 3 × 106 hA-MSCs/mL over a 7–11 day period (Figure 4).
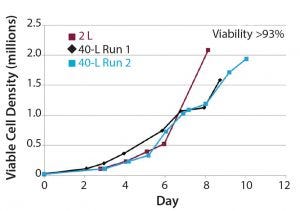
Figure 5: Comparison of 50-L, single-use bioreactor platform (black and blue) and 2-L stirred-tank bioreactor platform
The process was scaled up and transferred to a 50-L single-use bioreactor platform. We obtained reproducible growth profiles that were similar to the small-scale process that produced 2 × 106 hBM-MSCs/mL by day 10 (Figure 5). Cell viability was not compromised in the scaled-up bioreactor platform model. In a scaled-down bioreactor platform model for expansion of human pluripotent stem cells using single-use disposable vessels and microcarriers, cell yields of 4 × 106 cells/mL in 17 days were achieved (Figure 6).
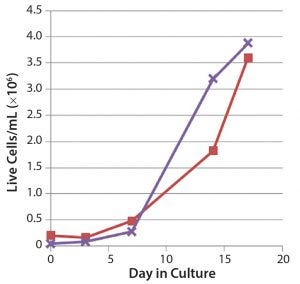
Figure 6: Human pluripotent stem cell expansion in scaleddown model of Lonza’s 3D bioreactor platform
Bioreactor Expansion Strategies
Feeding: To achieve optimal cell yields and high-quality final products, optimizing the feeding strategy for a culture expansion protocol is essential. Although in planar systems media typically are exchanged every two to three days, that may not be optimal or sufficient in a bioreactor system. In an optimized 3D culture, cell density is expected to increase at a faster proliferation rate than in planar culture, thus requiring more nutrients and possibly causing the accumulation of inhibitory products at a faster rate than for a 2D system. To balance cell requirements of maintaining adequate amounts of key growth factors in the media while ensuring noninhibitory levels of waste accumulation, 3D culture platforms might require more aggressive feeding strategies (e.g., fed-batch or perfusion) to ensure a low-stress culture environment for high-quality products. The accelerated growth rate and higher cell yield in most cases more than justify the increase in media use.
Monitoring: Some commercially available instruments can collect real-time growth kinetics, nutrient consumption, and metabolite concentration measurements without sample removal or product contact. As mentioned above, many bioreactor systems now incorporate pH and dissolved oxygen (DO) sensors into their disposable culture vessels, and some designs are working toward adding glucose and lactate disposable sensors as well. Advanced stainless-steel probes with powerful analytical capabilities can be incorporated into novel production platforms for the robust production of high-quality, active pharmaceutical ingredients. Those instruments can help prevent many costly process deviations by automating the culture process in a control feedback loop with the bioreactor controller. Such a design minimizes risk of batch contamination or loss due to improper sampling, feeding, and sterile welding required for sample removal and other operations.
Harvesting: Typically, three common harvesting methods can be used in cell therapy: sedimentation, filtration, and centrifugation. Sedimentation is a process in which vessel agitation is stopped, cells are allowed to settle, supernatant is removed, dissociation occurs, and the cell suspension is transferred to another vessel. That method is common in smaller scale, research, or preclinical trial processes.
For larger scale cultures, filtration — e.g., using disposable tangential-flow filtration (TFF) filters — can wash and concentrate cells and separate them from microcarriers. In this method, harvested materials are fed into a filter with pore sizes larger than the cells of interest, and the permeate stream passes through the membrane while waste and/or microcarriers are collected in the retentate stream. Over the past few years, TFF systems have become more adapted to cell therapy processes. Traditionally, the retention of cells (but not necessarily cell health) was the focus of the technology. However, filtration companies now offer modified products and protocols to maintain both cell viability and retention throughout harvest, concentration, and wash.
An additional cell separation technology, that also uses single-use components for closed-system harvest is the kSep system (Sartorius, Germany). In this system, the basic principle is to create a balance between centrifugal forces and flow rates for optimized product wash and harvest.
Some technologies can be programmed to harvest, concentrate, and wash cells automatically from the expansion vessel at optimal settings to maintain culture health at the highest possible yields. Some devices can automatically fill final product into suitable vials, also using disposables that can be directly connected to your harvest technology.
Which Is Right for Your Process?
Many technologies are available and currently being developed specifically for cell therapy. Although cell expansion and harvest yields are important, the most appropriate bioreactor platform is one that can produce a robust process for generation of high-quality active pharmaceutical ingredients, has batch-to-batch consistency, is manufactured at a clinically relevant production lot size, and allows a CoGs that will be reimbursed by payers to allow the patients that need these treatments to receive them.
References
1 Weng JY, et al. Mesenchymal Stem Cell As Salvage Treatment for Refractory Chronic GVHD. Bone Marrow Transplant 45(12) 2010: 1732–1740.
2 Lee JW, et al. A Randomized, Open-Label, Multicenter Trial for the Safety and Efficacy of Adult Mesenchymal Stem Cells After Acute Myocardial Infarction. J. Korean Med. Sci. 29(1) 2014: 23–31.
3 Weiss DJ, et al. A Placebo-Controlled, Randomized Trial of Mesenchymal Stem Cells in COPD. Chest 143(6) 2013: 1590–1598; doi: 10.1378/chest.12-2094.
4 Microcarrier Cell Culture: Principles and Methods. GE Healthcare Life Sciences: Little Chalfont, UK, 2005.
Erika McAfee is a scientist, and Sunghoon Jung is a senior scientist, research and technologies, and Eytan Abraham is director of emerging technologies, all at Lonza, Inc., 8830 Biggs Ford Road, Walkersville, MD 21793.
You May Also Like






