Large-Scale Purification of Factor-IX: Comparing Two Affinity Chromatography ResinsLarge-Scale Purification of Factor-IX: Comparing Two Affinity Chromatography Resins
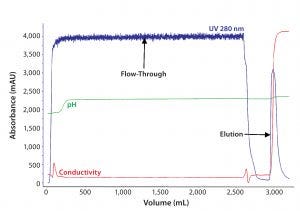
Figure 1: In the first chromatographic step of purification of F-IX from human plasma by anionexchange (AEX) chromatography, human plasma after cryoprecipitation was loaded onto an AEX resin, and F-IX was eluted with a 0.3M NaCl-step gradient.
Human clotting factor-IX (F-IX) is a glycoprotein that is essential for normal hemostasis (1). A deficiency of F-IX in human plasma is caused by an absence or functional mutation of the F-IX gene that expresses inactive F-IX in plasma. That leads to hemophilia B (“Christmas disease,” named after its first identified patient), a genetic disorder in which the blood-clotting cascade is disturbed (2, 3).
The structure and amino-acid sequence of F-IX are similar to those found in other vitamin-K–dependent glycoproteins. Apart from F-IX, these clotting factors include factor II (F-II), factor VII (F-VII), factor-X (F-X), and protein C. F-IX has an apparent molecular mass of 55,000–65,000 Da (4–8). Calculated from the amino acid sequence alone, its predicted molecular mass is 47,054 Da, with added carbohydrate content of about 17%. Because of acidic oligosaccharides (10 sialic acid residues per molecule), human F-IX has a low isoelectric point of 4.0–4.6.
Also known as “Christmas factor,” F-IX is a serine protease of the coagulation system (9). It belongs to the S1 peptidase family (10) and is produced as an inactive precursor (zymogen) (11). F-IX is processed to remove its signal peptide, then glycosylated and cleaved by factor XIa (intrinsic pathway) or factor VIIa (extrinsic pathway) to produce a two-chain form with chains linked by a disulfide bridge (1, 12, 13). When activated into factor IXa in the presence of Ca2+, membrane phospholipids, and a factor VIII cofactor, F-IX hydrolyses one arginine-isoleucine bond in factor X to form factor-Xa. The molecular action is inhibited by antithrombin (14).
The concentration of F-IX in human plasma is very low, amounting to 10 µg/mL in healthy adults (3). Administering whole blood or human plasma to correct F-IX deficiency leads to volume overloading and an overdose of other proteins. That creates a need to process plasma through methods such as chromatography to yield highly pure F-IX.
To correct this coagulation factor deficiency and prevent bleeding, replacement therapy with intravenously delivered, plasma-derived or recombinant F-IX concentrates is the cornerstone of managing patients with hemophilia B (12). Specific and viral-inactivated products are the sources of choice for these missing proteins, preferred over fresh-frozen plasma and prothrombin complex concentrates.
Various reports describe the purification of factor IX through use of the following affinity ligands: heparin, murine antihuman F-IX antibodies, metal chelate, or sulfated dextran (15–18). Such processes usually involve capture of the F-IX complex (containing factors II, VII, X, IX, protein C, proteins S, and other proteins from cryopoor plasma) on DEAE-Sephadex A-50 or DEAE-cellulose media (19). The DEAE eluate is purified further on an immobilized-heparin column (20, 21). Most of these methods yield low recovery of factor IX, however, with low specific activity.
We studied two affinity chromatography resins with high potential for selecting out F-IX from other plasma proteins. The base matrix of affinity chromatography resin A (GE Healthcare’s Heparin Sepharose 6 Fast Flow affinity resin) consists of highly crosslinked 6% agarose beads. Heparin is linked to the Sepharose matrix by reductive amination, which provides for very stable binding. Affinity resin B (IX Select resin) also has a base matrix of rigid agarose that works at high flow rates and with low back pressures, but a single-chain antibody fragment directed against F-IX is used as an affinity ligand (also from GE Healthcare). Affinity resin B enables efficient capture and intermediate purification of F-IX with high purity and yield.
Here we describe our use of anion-exchange (AEX) chromatography for capture of F-IX from plasma and subsequent purification using two different affinity chromatography resins. Use of affinity followed by hydrophobic-interaction chromatography (HIC) yields highly purified F-IX concentrate. The AEX resin separates F-IX along with other clotting factors that are eluted with higher ionic strengths (21). The process is designed such that viruses are inactivated by solvent–detergent (S–D) treatment in the initial steps before affinity chromatography. Tri-N-butylphosphate (TnBP) solvent and Tween-80 detergent are removed in the affinity chromatography step (21). Virus-retentive filtration is an additional method for removing small, nonenveloped adventitious viruses (20, 22–24).
In 2016, Pilli et al reported using these same affinity resins to purify murine F-IX (25). But we are the first to use it for purifying F-IX at large scale from human plasma. We compare the two affinity chromatography resins for selective purification of F-IX herein, having produced highly pure (96%) F-IX drug substance from human plasma with a specific activity of 250 ± 30 IU/mg for patients with hemophilia B.
Materials and Methods
We obtained fresh-frozen plasma in polyvinyl bags from blood banks approved by the US Food and Drug Administration (FDA) and registered with the National AIDS Control Organization under the government of India. We used standard FDA-approved kits from Abbott Molecular to perform nucleic acid testing for hepatitis-B virus (HBV), hepatitis-C virus (HCV), and human immunodeficiency virus-I (HIV-I). Antibody testing kits for HIV-I, HIV-II, and HCV came from Bio-Rad Laboratories, and an HBV kit came from Diagnostic Bioprobes. AEX, affinity, and HIC resins for F-IX purification came from GE Healthcare, as did the columns we used.
For our F-IX potency assay, we used factor diluent, cleaning solution, SynthASil buffered reagent, F-IX–deficient plasma, and calibration plasma from Instrumentation Laboratories. For sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), we used a standard protein molecular weight marker and Quick Start Bradford 1× dye reagent for total protein estimation from Bio-Rad Laboratories. Bovine serum albumin and chemicals for SDS-PAGE analysis came from MilliporeSigma, and glycine and glycerol came from Merck.
Plasma was clarified using 3.2-m2, 6-µm Microfilt NA600 filter; F-IX bulk was filtered through a 0.2-µm sterile filter (Pall Life Sciences). We used commercially available F-IX as a reference standard. All other chemicals came from Merck and MilliporeSigma.
Plasma Testing: Every unit of plasma we used was nucleic-acid tested for HBV, HIV-I, and HCV. All units that tested negative were pooled and used for the purification study. Plasma also was tested for the presence of antibodies against HIV-I, HIV-II, HBV, and HCV. Again, only those testing negative were used.
Protein Concentration: Total protein was determined by the Bradford method using bovine serum albumin (BSA) as a standard. Sample readings taken at 595 nm using a plate reader were subtracted from the Bradford reagent blank. We determined the F-IX sample concentration based on optical density at 595 nm using a BSA standard curve.
Electrophoretic Methods: We used nonreducing conditions for SDS–PAGE. Electrophoretic separations were carried out on 10% separating gel (1.5 M Tris-Cl, pH 8.8) and 4% stacking gel (0.5 M Tris-Cl, pH 6.8). The running buffer was Tris–glycine–SDS buffer (pH 8.3). We ran the gel under a constant 200 volts for 45 minutes in a Bio-Rad Laboratories Miniprotean Tetra cell assembly. Silver staining was based on manufacturer’s recommendations.
Protein Purity (Analysis By Size-Exclusion High-Performance Liquid Chromatography, SE-HPLC): For analytical SE-HPLC, we separated samples with 5-µm G3000SWXL TSK gel, 30 cm × 7.8 mm i.d., from Tosoh Biosciences. The column was connected to an Agilent Technologies 1260 Infinity system in quaternary gradient mode. We used phosphate buffer with saline at pH 7.0 as the mobile phase. Samples were normalized to 0.1 mg/mL in the mobile phase before injecting 100 µL into the instrument set for a flow rate of 0.5 mL/min and absorbance at 280 nm.
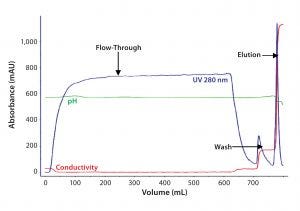
Figure 2A: For the second step in Experiment One, eluate from AEX chromatography after S–D treatment was loaded on an affinity resin A column. F-IX was eluted with a 0.5M NaCl– step gradient.
Factor-IX Potency Analysis: We tested the potency of our purified F-IX using an ACL Elite Pro automatic coagulometer from Instrumentation laboratories for a single-stage coagulation assay.
Experimental Procedure: Factor IX was purified from fresh-frozen human plasma that tested negative for HBV, HIV, and HCV. We manually cut 200 L of plasma frozen at –40 °C into 250-L polyvinyl bags, then pooled, thawed, and centrifuged the plasma (using a Westfalia Separator system) to prepare cryopoor plasma according to Brummelhuis’ method (12) with modifications that have been described elsewhere (10).
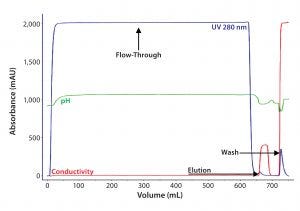
Figure 2B: For the second step in Experiment Two, eluate from AEX chromatography after S–D treatment was loaded on an affinity resin B column. F-IX was eluted with 2M MgCl2.
Briefly, the initial step is a solid-phase extraction of the cryopoor plasma with an AEX chromatographic resin (DEAE Sepharose Fast Flow resin from GE Healthcare). That followed clarification of the cryopoor plasma. Then the elution was subjected to S–D treatment with 1% (w/v) Tween 80 and 0.3% (w/v) TnBP for six hours at 27 °C. Selective F-IX separation was based on affinity chromatography resins A (Heparin Sepharose 6 Fast Flow) and B (IXSelect), both also from GE Healthcare. In one experiment, we collected the eluate from affinity resin A and analyzed it for purity, activity, and protein content using SDS-PAGE, potency, and total-protein assays.

Figure 3: 10% silver-stained sodium-dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) profile of affinity resin eluates under nonreducing conditions. Lane 1: Standard molecular weight markers in kDa (4 µL) Lane 2: Resin A input Lane 3: Resin A flow-through Lane 4: Resin A wash Lane 5: Resin A elution Lane 6: Resin B input Lane 7: Resin B flow-through Lane 8: Resin B wash Lane 9: Resin B elution Lane 10: Reference product
In the second experiment, we loaded treated eluate onto affinity resin B and analyzed the eluted protein of interest for purity, activity, and protein content using SDS-PAGE, potency, and total-protein assays. For the final step in chromatographic purification (polishing) we used a HIC resin (Phenyl Sepharose 6 Fast Flow from GE Healthcare) and virus filtered the eluate with an Asahi-Kasei Planava 20N filter followed by a 0.2-µm sterile filter (MilliporeSigma). Thus purified, F-IX was collected and analyzed with SDS-PAGE, SE-HPLC, potency, and total-protein assays.
Results and Discussion
The first step in F-IX purification influences the subsequent processes. The capture step includes an AEX resin that effectively binds clotting factors that depend on vitamin K (FIX, FII, FVII, and FX), because of their similar binding properties, as well as other high– and low–molecular-weight proteins. With increased conductivity in the elution buffer, bound proteins desorb from the column (Figure 1). Specific activity of F-IX in the AEX eluate was 1.2 ± 0.3 IU/mg of protein.
For intermediate purification, we compared affinity ligands in affinity chromatography resins A and B by loading eluate from the AEX resin onto them simultaneously. Figures 2A and 2B show comparative chromatographic purification profiles for these affinity resins. From those profiles, we observed that the B ligand is more specific for F-IX and therefore binds much less total protein.
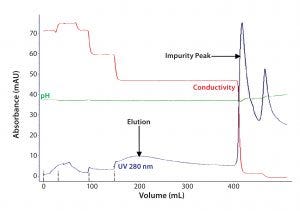
Figure 4A: For the third step in Experiment One, eluate from affinity chromatography resin A was loaded onto a HIC column. F-IX was eluted with 0.4M salt concentration (step gradient).
We analyzed eluates from both resins by nonreducing SDS-PAGE. From that, we observed that the elution fraction from affinity resin B (Figure 3, lane 9) showed fewer high–molecular-weight impurities than that from affinity resin A (Figure 3, lane 5). That indicates a higher level of purity, with a major band at an observed molecular weight of about 65,000 Da in affinity resin B eluate. F-IX bands observed at about 65,000 Da in both affinity elutes were comparable to that of a F-IX reference product measured by SDS-PAGE using two-step purification (AEX followed by affinity chromatography). The specific activity of F-IX was determined to be 35 ± 5 IU/mg of protein using affinity resin A compared with 160 ± 20 IU/mg of protein obtained with affinity resin B. So affinity resin B showed about 4.5× better performance than affinity resin A.
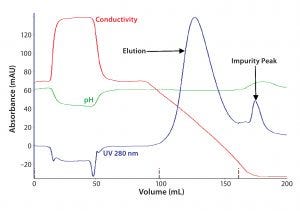
Figure 4B: For the third step in Experiment Two, elute from affinity chromatography resin B was loaded onto a HIC column. F-IX was eluted with a linear gradient of 0.6-0M salt concentration.
For removing trace impurities and/or leachables that could have come from the affinity resins, we introduced a final polishing step using HIC. Figures 4A and 4B show elution profiles for the affinity resin eluates loaded onto HIC.
Figure 5B shows a purified F-IX protein band from affinity resin B followed by HIC, with an insignificant amount of impurities as measured by SDS-PAGE compared with affinity resin A followed by HIC (Figure 5A). The purified F-IX band corresponds to the main band of the F-IX reference product. The final purification step yielded enriched and pure F-IX protein with a specific activity of 250 ± 30 IU/mg from a process using affinity resin B, which is 2.5× higher than from the purification process using affinity resin A.
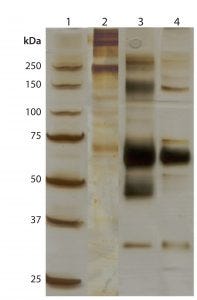
Figure 5A: 10% silver-stained SDS-PAGE profile of HIC samples after affinity chromatography resin A under nonreducing conditions (with 25-µL sample loading).
Lane 1: Standard molecular weight markers in kDa (4 µL) Lane 2: Resin A elution Lane 3: Reference product Lane 4: HIC elution
Using SE-HPLC, we studied high– and low–molecular-weight impurities in eluate from the final step of HIC chromatography after both affinity resin A and B as an intermediate chromatography step. Our results indicated highly purified (96%) F-IX product from the resin B process compared with 84% purity from the resin A process. Based on these above findings and comparative analysis, we chose affinity resin B for our F-IX purification process.
Selection Success
Affinity chromatography provides a rapid and effective tool for selective purification of the molecule of interest from a crude mixture of proteins. After comparative evaluation of two affinity resins for human F-IX, we observed that resin B showed a 10–15× higher dynamic binding capacity (DBC), better purity by SE-HPLC, and higher selectivity than resin A. Higher productivity was achieved using resin B in our F-IX purification process than from resin A in the same process design. Thus we have successfully demonstrated the use of a specific single-chain antibody fragment directed against F-IX for large-scale purification of highly concentrated F-IX from a heterogeneous mixture of proteins in a plasma pool.
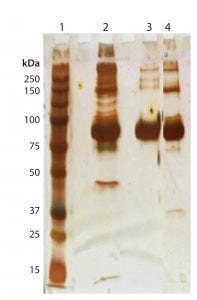
Figure 5B: 10% silver-stained SDS-PAGE profile of HIC samples after affinity chromatography resin B under nonreducing conditions (with 25-µL sample loading)
Lane 1: Standard molecular weight markers in kDa (4 µL) Lane 2: Resin B elution Lane 3: Reference product Lane 4: HIC elution
Acknowledgments
We acknowledge the support and cooperation of GE Healthcare India for this project and would like to thank the management of Intas Pharmaceuticals for providing us with the resources necessary to execute this work.
References
1 Pock K, et al. Characterization of Clotting Factor IX in Plasma-Derived Preparations By Electrophoretic Techniques. J.
Chromatogr. A 921(1) 2001: 57–67; doi:10.1016/S0021-9673(01)00614-8.
2 Davie EW, Fujikawa K, Kisiel W. The Coagulation Cascade: Initiation, Maintenance and Regulation. Biochem. 30(43) 1991: 10364–10370; doi:10.1021/bi00107a001.
3 Curling J. Integrating New Technologies for Plasma Blood Fractionation. BioPharm September 2002: 16–26.
4 DiScipio RG, et al. A Comparison of Human Prothrombin, Factor IX (Christmas Factor), Factor X (Stuart factor), and Protein S. Biochem. 16(4) 1977: 698–706.
5 Suomela H. Human Coagulation Factor IX Isolation and Characterization. Eur. J. Biochem. 71, 1976: 145–154.
6 Buchacher A, et al. High-Performance Capillary Electrophoresis for In-Process Control in the Production of Antithrombin III and Human Clotting Factor IX. J. Chromatogr. A 802(2) 1998: 355–366; doi:10.1016/S0021-9673(97)01184-9.
7 Osteurud B, et al. Human Blood Coagulation Factor IX. J. Biol. Chem. 253, 1978: 5946–5951.
8 Velander WH, et al. Process Implications for Metal-Dependent Immunoaffinity Interactions. Biotechnol. Prog. 5, 1989: 119–125.
9 Huang CC, et al. Preparation of Factor IX. US Patent 6043215A: June 1995.
10 Judith R, Peter MW, Robert RD. Inhibition of Human Factor IXa By Human Antithrombin. J. Biol. Chem. 250(23) 1975: 8883–8888.
11 Chung KS, et al. Purification and Characterization of an Abnormal Factor IX (Christmas Factor) Molecule. J. Clin. Invest. 62(5) 1978: 1078–1085; doi:10.1172/JCI109213.
12 Lowe G. Factor IX and Thrombosis. Bri. J. Haematol. 115(3) 2001: 507–513; doi:10.1046/j.1365-2141.2001.03186.x.
13 Mackman N, Tilley RE, Key NS. Role of the Extrinsic Pathway of Blood Coagulation in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 27(8) 2007: 1687–1693; doi:10.1161/ATVBAHA.107.141911.
14 Giannelli F. The Genetics of Blood Coagulation and Haemostasis. Haemophilia and Other Inherited Bleeding Disorders. Rizza CR, Lowe GDO Eds. Sauders: London, 1997: 43–86.
15 Burnouf T, Radosevich M. Affinity Chromatography in the Industrial Purification of Plasma Proteins for Therapeutic Use. J. Biochem. Biophys. Meth. 49(1–3) 2001: 575–586.
16 Osterud B, Flengsrud R. Purification and Some Characteristics of the Coagulation Factor IX from Human Plasma. Biochem. J. 145(3) 1975: 469–474; doi:10.1042/bj1450469.
17 Husi H, Walkinshaw MD. Purification of Factor X By Hydrophobic Interaction Chromatography. J. Chromatogr. B: Biomed. Sci. Appl. 755, 2001: 367–371.
18 Choudhury S, et al. Mini-Review on “A Novel One-Step Purification of Mouse Factor IX.” J. Rare Dis. Res. Treat. 1(2) 2016: 8–10.
19 Josic D, et al. Manufacturing of a Prothrombin Complex Concentrate Aiming at Low Thrombogenicity. Thromb. Res. 100(5) 2000: 433–441.
20 Hoffer L, et al. Improved Virus Safety and Purity of a Chromatographically Produced Factor IX Concentrate By Nanofiltration. J. Chromatogr. B 669(2) 1995: 187–196; doi:10.1016/0378-4347(95)00107-T.
21 Hoffer L, Schwinn H, Josic D. Production of Highly Purified Clotting Factor IX By a Combination of Different Chromatographic Methods. J. Chromatogr. A 844(1–2) 1999: 119–128; doi:10.1016/S0021-9673(99)00348-9.
22 Dileo AJ, Allegrezza AE, Builder SE. High Resolution Removal of Virus from Protein Solutions Using a Membrane of Unique Structure. Biotechnol. 10, 1992: 182–188; doi:10.1038/nbt0292-182.
23 Burnouf-Radosevich M, et al. Nanofiltration: A New Specific Virus Elimination Method Applied to High-Purity Factor IX and Factor XI Concentrates. Vox Sang. 67(2) 1994: 132–148.
24 Brummelhuis HGJ. Preparation of the Prothrombin Complex. Methods of Plasma Protein Fractionation. Curling JM, Ed. Academic Press: London, 1980; 117–128.
25 Pilli VS, et al. A Novel One-Step Purification of Mouse Factor IX. Thromb. Res. 139, 2016: 125–126; doi:10.1016/j.thromres.2016.01.013.
Amit Pawar ([email protected]), Sheetal Dolia ([email protected]), and Sumita Bardhan ([email protected]) are senior research scientists; and corresponding author Suma Ray, PhD, is associate vice president of plasma operations at Intas Pharmaceuticals Ltd. (Celestial Division), Plot No. 496/1/A & B, Sarkhej-Bavla Highway, Village Matoda, Taluka Sanand, Ahmedabad, Gujarat–382213, India; 91-02717-662103; [email protected]; www.intaspharma.com.
You May Also Like






