Selective and Flexible Chromatography Media: Improving Biopharmaceutical Operational EfficienciesSelective and Flexible Chromatography Media: Improving Biopharmaceutical Operational Efficiencies

Along with increased selectivity, mixed-mode and multimode media offer significant potential operational cost savings by eliminating intermediate purification steps that require additional time, materials, equipment, and personnel.
Continuing development in protein and peptide engineering have produced a broad range of new biological products with improved therapeutic and diagnostic potential. In the development pipeline, more than 900 biologic products target more than 100 diseases (1). Increased manufacturing complexities caused by closely related impurities and requirements to improve process efficiencies and reduce operating costs highlight the need for new approaches in protein purification.
Platform-based chromatographic approaches have been successfully applied in separating and purifying monoclonal antibody (MAb) products. But the next generation of therapeutic proteins — e.g., tetravalent bispecific antibodies, bispecific T-cell engagers (BiTes), nanobodies, and antibody fragments — presents different obstacles for downstream process developers to overcome (2). Product- and process-related impurities — including host cell proteins, aggregates, and charge and glycosylation variants — are persistent and often difficult to separate from a protein of interest. Many process developers are exploring mixed-mode and multimode chromatographic media to achieve those separations.
Here we explore the mechanisms by which mixed-mode (hydrophobic and anionic exchange) and multimode (cation and anion exchange) interactions can improve selectivity, efficiency, and operational flexibility over those of standard purification sequences. We also examine how innovative process chromatography media can reduce the number of steps required for purification, improving downstream process efficiency and yield while potentially lowering costs.
Challenges of New Biopharmaceutical Production
The biopharmaceutical landscape is becoming more complex and diverse, with an increasing number of MAb-based recombinant protein such as diabodies (bispecific antibodies) currently in development. These molecules have more complex structures than earlier generations of MAbs and other proteins.
Biopharmaceutical manufacturers strive to reduce (and eliminate to the greatest extent possible) variability in their biomanufacturing processes, thus minimizing drug product heterogeneity. However, production of these precisely targeted drug molecules also can yield byproducts that are very closely related to them but that chemically or biologically have no therapeutic value.
Even as the biopharmaceutical industry faces those new purification challenges, it also is experiencing a strong drive to control overall processing costs without sacrificing product yield or therapeutic value. Leveraging the power of new process chromatography technologies is one principal option for accomplishing both objectives.
Chromatography Selectivity and Efficiency
Significant efforts have been made to improve process chromatography technology and address biopharmaceutical purification challenges. Researchers are focusing their efforts on two critical performance parameters in particular: selectivity and efficiency.
Selectivity is often the dominant criterion for companies considering chromatographic media, and they may choose from multiple approaches to achieve it. Traditional approaches involve modifying process conditions of a generally available medium or adding multiple chromatography steps to achieve a desired purity level. Logically, adding steps also adds process time and cost.
Separating undesired glycosylated molecules, charge variants, heterogeneous molecules (process variants), and aggregates presents major challenges in antibody purification (3). They are likely to have limited differential binding to traditional ion exchangers and can
coelute with products. The traditional solution to that problem is to implement multiple separations based on simple ion-exchange chromatographic media.
Advanced methods for achieving effective selectivity come with new ligand chemistries that are engineered to provide very precise, selective interactions with targeted proteins. This approach increases selectivity while reducing the number of processing steps required, which helps biomanufacturers control both process complexity and costs.
Targeted Affinity: To meet the purification requirements of increasingly complex biopharmaceutical molecules, process chromatography suppliers are developing new alternatives to traditional ion-exchange chromatography media. These alternatives include mixed-mode, multimode, and targeted affinity media.
Targeted affinity chromatographic media are based on ligands that are tailored to interact with specific proteins, offering high selectivity for a given drug molecule. This technology offers the selectivity and binding-capacity advantages of standard affinity media, but it can be a time-consuming method if implemented for every new type of molecule. In addition, the targeted-affinity approach presents cost, scalability, lead time, supply capacity, and supply chain transparency challenges.
Both mixed-mode and multimode approaches offer advantages for biopharmaceutical producers working to balance their goals of improved selectivity and chromatography efficiency with the parallel efforts to keep process complexity and costs under control.
Mixed-Mode Chromatography
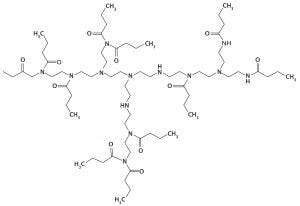
Figure 1: Mixed-mode ligand structure — mixed-mode media offer more interaction possibilities with targeted drug molecules.
Mixed-mode chromatography media are based on ligands that offer two or more interaction possibilities with a target drug molecule (Figure 1). Selectivity is modulated by operating conditions such as pH and/or conductivity.
The mixed-mode approach has been effective and more productive in applications such as intermediate and polishing steps for purifying proteins based on differential salt-induced hydrophobicity. The advantage with this approach is that the same media can be used for different purification steps if modulated by solution conditions.
For example, selectivity can be manipulated and optimized by altering feed conditions, which modifies interactions between hydrophobic and ion-exchange groups present on media surfaces. To optimize selectivity for a particular biomolecule, feed conditions (such as multiple buffers) or multiple elution steps may be needed to activate different modes. That can add development time, could add a buffer step, and might negatively influence further downstream processes (e.g., by increased conductivity and requiring additional dilution).
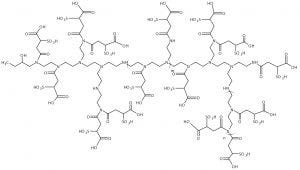
Figure 2: Multimode ligand structure — multimode resins can simultaneously interact with different sites or regions of a protein molecule.
Some advanced mixed-mode resins offer ligand chemistries that provide for multiple sequential interactions during the course of a normal chromatographic process. These chromatography media offer the opportunity to overcome some of the limitations identified for mixed-mode technology.
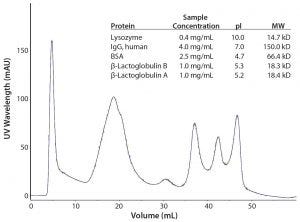
Figure 3, Table 1: Separation of closely related proteins using multimode anion-exchange chromatography medium. Column: 0.77 × 10 cm Buffer A: 50 mM TRIS pH 8.0 Buffer B: Buffer A plus 1 M NaCl, pH 8.0 Linear Gradient: from 0 to 100% B in 10 CV Flow Rate: 1.2 mL/minute Injection Volume: 1.5 mL
Multimode Chromatography
Multimode resins use ligands that can interact simultaneously with different sites or regions of a protein molecule (Figure 2). These media offer a more sophisticated and potentially efficient technology than both traditional ion-exchange media and mixed-mode media for process chromatography. Rather than requiring multiple chromatographic purification steps, the simultaneous interactions make it possible for biomanufacturers to separate very closely related proteins in a single step (Figure 3, Table 1).
It is possible to have primary, secondary, and tertiary interactions all happening at the same time. Such simultaneous purification can occur without requiring additional intermediate steps such as buffer exchange, buffer titration, or dilution. And those require time, add materials cost, and pose the risk of introducing additional variables into a downstream process.
These more advanced chromatography media could facilitate significant process efficiencies. Given the challenges associated with manufacturing the complex next-generation protein products now in development, thorough consideration of both mixed-mode and multimode chromatography is warranted.
Cost Modeling
Process chromatography is one of the most expensive steps in biopharmaceutical production. Downstream MAb production can represent 50–60% of total processing costs. This situation has led to expanded applications of cost modeling for helping companies manage those costs and guide managers in decision-making.
In the past, most cost analyses focused on the actual cost of chromatography columns and media in isolation. Recently, however, decision makers are pursuing a more holistic approach that takes into account multiple dynamics. Most biopharmaceutical manufacturers can consult multiple years’ worth of historical process information that helps them analyze the dynamics between yield and throughput.
The advent of mixed-mode and multimode media presents significant potential savings from reducing process chromatography steps in downstream processing. The increased selectivity and efficiency these technologies gives biomanufacturers the possibility of eliminating process steps (reducing, for example, three purification steps to two). That translates into significant operational cost savings. In addition, multimode chromatographic media can make it possible to eliminate intermediate steps that require additional time, materials, equipment and personnel.
Tools for Process Improvement
The latest generations of both mixed-mode and multimode chromatography media offer effective solutions for biopharmaceutical processing. The advances these technologies offer — especially in terms of their high selectivity — make them extremely useful tools. They efficiently remove product- and process-related impurities, especially the byproducts that are chemically or biologically very closely related to target molecules.
Choosing the right media for a given molecule and process involves several key considerations: e.g., cost, selectivity, yield, and efficiency. A better understanding of the ways in which different mixed-mode and multimode media operate — along with key technical characteristics of the latest generation of chromatography media — can provide the most effective framework for making chromatography choices that will help biomanufacturers improve process efficiency, yield, and productivity.
Reference
1 Medicines in Development: Biologics Overview Report. Pharmaceutical Research and Manufacturers of America: Washington, DC, 2013; http://phrma.org/sites/default/files/pdf/biologicsoverview2013.pdf.
2 Elvin JG, Couston RG, van der Walle CF. Therapeutic Antibodies: Market Considerations, Disease Targets, and Bioprocessing. Int. J. Pharmaceut. 440, 2013: 83–98.
3 Brorson K, Jia AY. Therapeutic Monoclonal Antibodies and Consistent Ends: Terminal Heterogeneity, Detection and Impact on Quality Curr. Opin. Biotechnol. 30, 2014: 140–146.
Further Reading
Guo W, et al. Removal of IgG Aggregates Using Anion-Exchange Chromatography in Flow-Through Mode. PREP 2011: Boston, MA.
Thiyagarajan B, Lohse K, Deorkar N. Understanding Chromatographic Media Ligand Density. BioProcess Int. 9(10) 2011, 56–58.
Guo W, et al. High Selective One-Step Purification of IgG from Serum Using Polymeric Mixed-Mode Cation-Exchange Media. HPLC 2009: Dresden Germany.
Corresponding author Nandu Deorkar, PhD, MBA, is vice president of research and development; and B. Thiyagarajan, PhD, is biopharmaceutical group leader for Avantor Performance Materials, 3477 Corporate Parkway, Suite #200, Center Valley, PA 18034; 1-610-573-2600.
You May Also Like






