Synthetic Biology for Adapting CHO Cells to Challenging BioprocessesSynthetic Biology for Adapting CHO Cells to Challenging Bioprocesses
Figure 1: Synthetic biology opens up novel cell functions by means of genetic modulation (FIGURE GENERATED USING BIORENDER SOFTWARE).
Biopharmaceuticals are produced mainly by Chinese hamster ovary (CHO) cell lines, for which advances in protein formats, bioprocesses, and bioprocess control are introducing novel challenges (1). Thus far, those challenges have been tackled either by technical innovations and media optimization or by advances in host-cell engineering (2, 3). Some technical innovations bring further challenges, such as those related to the compatibility of CHO cultures with highly automated bioprocesses and continuous high-density culture modes (4). With regard to host-cell engineering, most optimization efforts have been based on rational, one-dimensional approaches focusing on conventional single-gene overexpression, knockout, or knockdown (2). Synthetic biology offers a spectrum of molecular biology methods for redesigning organisms to useful purposes by engineering them to have new abilities. That enables developers to harness the power of nature in solving problems for medicine or biomanufacturing.
We explored synthetic biology for cell engineering and present here a combination of approaches not only to tackle some performance issues, but also to adapt and optimize CHO cells to address new bioprocess-related challenges. To that end, we exploited hypoxia as a means of adapting cells to continuous, high-density biomanufacturing (5) and developed a sensor cell line for automated bioprocess control (6) (Figures 1–3).
Continuous Bioprocessing
Specialized Cells to Cope with and Exploit Hypoxia: Oxygen deficiency can be a challenging parameter in cell cultures, leading to unfavorable cellular behavior and significantly deteriorated performance (7, 8). In production of biotherapeutic recombinant proteins, production cell lines encounter adverse hypoxic conditions during both classical batch fermentations and perfusion processes, in which high cell densities or volumes of >5,000 L are used to deliver high product titers (8–12). A sufficient oxygen supply of at least 10–15% pO2 is crucial to maintain an optimal energy metabolism for cellular functions such as growth, protein production, and autophagy (5, 13–15).
However, CHO production cell lines currently are not optimized for efficient protein production under hypoxic conditions (16–18). That places great demand on cell engineering to adapt or improve this dominant production-cell system. Many studies have focused on the molecular improvement of CHO cells using engineering approaches to delete detrimental or overexpressing favorable genes (19–26). However, hypoxia adaptation/exploitation has not been addressed in published studies so far.
In nature, mammalian cells use response elements to sense and react to hostile environmental conditions (27–29). For example, oxygen deficiency leads to stabilization of hypoxia-inducing factor 1α (HIF-1α), which dimerizes with cofactor HIF-1β to interact with hypoxia response elements (HREs). The subsequently induced expression of protective proteins such as erythropoietin (EPO) or vascular endothelial growth factor A (VEGF-A) help to perpetuate cellular physiological functions (30–33). By harnessing this natural oxygen sensing system, we established a novel hypoxia-responding cell system in CHO production cells based on HRE elements and cellular oxygen-sensing proteins (5). That opened the possibility for substantial improvement of recombinant protein production under hypoxic process conditions using a vector-based system in industrial settings, without time- and cost-intensive host-cell modifications.
Establishing this cell system began validating conservation of the hypoxia-responsive pathway in CHO cell lines (5). Polymerase chain reaction (PCR) and Western blot analysis were used to verify the expression of key pathway factors, including HIF-1α and HIF-1β, in CHO production cells. For construction of hypoxia-responsive vectors to drive protein expression selectively under hypoxic culture conditions, we used human-origin HRE building blocks, including the hypoxia-sensitive VEGF-A (29).
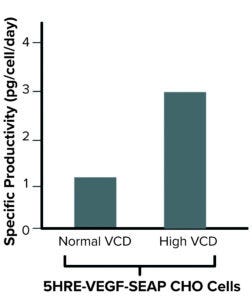
Figure 2: Exploitation of hypoxia increases specific productivity in highdensity cultivation upon stable expression of 5HRE-VEGF-SEAP in Chinese hamster ovary (CHO) cells (FIGURE GENERATED USING BIORENDER SOFTWARE).
We cloned a succession of five HRE-VEGF elements into an expression vector upstream of a full cytomegalovirus (CMV) promotor to drive expression of the recombinant model protein, secreted embryonic alkaline phosphatase (SEAP). Stable cell pools expressing SEAP under the control of a full CMV with 5HRE-VEGF elements were generated (CHO-5HRE-VEGF-SEAP). In a perfusion-mimicking bioprocess, cells under very high density demonstrated a significant increase in specific productivity of ~2.7-fold compared with normally cultured cells (Figure 2).
We also explored the system’s utility for improving production capacity of CHO cells even in classical process modes. We applied an oxygen downshift in combination with a classical temperature downshift during batch culture of CHO-5HRE-VEGF-SEAP cells to induce the vector system further. By contrast with a temperature shift alone, the downshift of both oxygen and temperature induced SEAP expression to a significantly stronger extent, resulting in a nearly twofold higher specific productivity.
Those data illustrate the potential of an oxygen-responsive vector system to enable significantly increased productivity under high-density culture conditions and after oxygen shifting during classical culture conditions. Establishment and use of specialized cells for specific bioprocess conditions — here exemplified by cells generated to cope with and exploit hypoxia — is an attractive approach to adapting CHO production cells for addressing novel production challenges.
Automated Bioprocess Control
Sensor Cell Line for In-Line Monitoring: Optimized bioprocess conditions are essential to ensuring strong cell growth, high productivity, and good product quality during industrial biomanufacturing (34). Oxygen deficiency and hyperosmolality are among the most critical process conditions that require continuous monitoring. In-line, on-line, and off-line monitoring systems available for bioprocessing include, for example, off-line osmolality sensors and pO2 probes for monitoring oxygen availability within bioreactors. When off-line measurements are used, however, the risk of contamination is increased by sampling procedures, and acquisition of periodical data points requires additional hands-on time. Moreover, because adverse culture conditions are not communicated autonomously by cells in culture, operators must define targeted values for pO2 and osmolality, which demands extensive testing to explore optimal conditions for each generated cell line.
Our aim was to generate a novel sensor cell line that autonomously reports unfavorable cultivation conditions at the molecular signal level to operators during bioprocessing. This would simplify measurements and improve insights into the modulation of intracellular physiological stages by real-time, in-line monitoring. To establish a genetic sensing system for bioprocess control in mammalian production cells, we again focused on synthetic biology using transcriptional sensors for cellular stress (6).
As mentioned, oxygen limitation is sensed within eukaryotic cells by binding of transcription factors to HRE elements, which we used to construct hypoxia-responsive vectors that selectively drive protein expression under hypoxic conditions. Next, to create hypoxia-monitoring and -sensing cell lines, we constructed expression vectors linking five HREs to a minimal CMV promoter that is not active by itself, thus ensuring expression of a marker protein (destabilized green fluorescent protein, dGFP) under only hypoxic conditions. After stable transfection of expression vectors, our engineered CHO cells showed increased fluorescence signaling after oxygen-limiting cultivation.
To broaden the applicability of the CHO sensor cell line, we next explored the creation of a synthetic multiplex sensor system. For bioprocess monitoring, osmolality is an additional important parameter to ensure optimal growth and productivity. In mammalian cells, it is sensed mainly by phosphorylation states and expression levels of nuclear factor of activated T-5 (NFAT5) cells (35, 36). During hyperosmolality, NFAT5 mRNA is stabilized, leading to higher amounts of NFAT5 protein within the cells. Phosphorylated NFAT5 enters nuclei and activates gene expression as a transcription factor by binding to osmolality-response elements (OREs) (37).
After verifying both the presence and functionality of that crucial factor for sensing osmolality in CHO cells, we constructed vectors that follow the same principle as above by linking a minimal CMV promoter to seven OREs that in this case drive the expression of another destabilized marker protein (blue fluorescent protein, FKBP-BFP). With NaCl addition, stably transfected cell pools showed up to a ninefold increase in FKBP-BFP signal compared with isotonic-cultured cells.
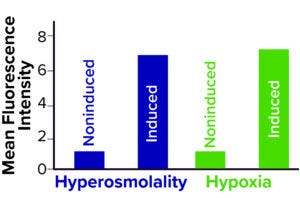
Figure 3: Hypoxia- and osmolalitysensing CHO cell lines were successfully induced upon NaCl addition and reduction of pO2 (FIGURE GENERATED USING BIORENDER SOFTWARE).
Next, to multiplex the inducible reporter systems to sense both hypoxia and hyperosmolality simultaneously, we transfected both vector systems stably into CHO cell lines and subsequently validated the functionality of the resulting synthetic sensing system in batch fermentations. Here, induced hyperosmolality and hypoxia were sensed and reported by the expression of FKBP-BFP and dGFP, respectively (Figure 3). That demonstrated the functionality of our sensor cell lines and the possibility of multiplexing response elements to monitor multiple cultivation conditions simultaneously. Finally, we attested the applicability of our CHO multiplex sensor cell line in a fully automated in-line monitoring system to observe the cellular state close to real-time using microscopy.
Implications and Potential
Complex biotherapeutics are produced mainly using CHO cells cultured in large-scale industrial bioreactors (38). To obtain ideal bioprocess conditions that enable strong cell growth, high product titer, and good product quality, the cells have to be adapted to challenging conditions requiring constant monitoring of critical parameters (34). To date, such challenges including high-density perfusion mainly have been tackled through media developments and technical innovations (4, 39). For high-cell density cultivation, popular solutions have included increased agitation and gassing rates, which entail drawbacks such as elevated shear stress, costs, and toxicity (40–42). By contrast, no cell-engineering approaches yet have been undertaken for adapting CHO cells to novel continuous bioprocessing conditions.
Our novel cell-engineering system to improve protein expression under hypoxic conditions encountered during high-density cultivations is, to our knowledge, the first attempt to adapt CHO cells to continuous bioprocess conditions by exploiting adverse conditions encountered during this bioprocessing format. Because it is vector-based and easy to integrate into existing expression vector systems, our system is applicable without complex or cost-intensive host-cell modifications. Thus, perfusion processes could benefit substantially from this system by exploiting induced hypoxia.
To tackle problems of on- or off-line bioprocess monitoring, we created a sensing cell line that detects adverse process conditions and expresses reporter proteins. We used hypoxia and osmotic response elements to control expression of destabilized fluorescent reporters as detectable markers for hypoxia and osmolality. That provided a stable CHO cell line that could report bioprocess conditions virtually in real time.
Because free fluorescent proteins could be problematic for downstream processing and therefore might represent an issue for regulatory authorities, we view the strength of our cell system to be primarily for modeling applications. With the option of integrating further response elements into the reporter cell line, our system could become key to development of next-generation bioprocesses. With the fluorescent feedback mechanism, operators can rely on easy-to-read and virtually real-time data rather than time-consuming sampling procedures followed by comprehensive analytics.
Synthetic biology is an attractive tool for adapting cell lines to novel bioprocess challenges. Our demonstration of engineered exploitation of hypoxia and osmotic response elements for improved bioproduction and monitoring thereof highlights that adverse process conditions can be converted into productive traits to be resolved virtually in real time. Thus, the synthetic cellular system enables application of novel bioprocessing strategies for optimized production.
References
1 Tihanyi B, Nyitray L. Recent Advances in CHO Cell Line Development for Recombinant Protein Production. Drug Discov. Today Technol. 38, 2020: 25–34; https://doi.org/10.1016/j.ddtec.2021.02.003.
2 Fischer S, Handrick R, Otte K. The Art of CHO Cell Engineering: A Comprehensive Retrospect and Future Perspectives. Biotechnol. Adv. 33(8) 2015: 1878–1896; https://doi.org/10.1016/j.biotechadv.2015.10.015.
3 Ritacco FV, Wu Y, Khetan A. Cell Culture Media for Recombinant Protein Expression in Chinese Hamster Ovary (CHO) Cells: History, Key Components, and Optimization Strategies. Biotechnol. Prog. 34(6) 2018: 1407–1426; https://doi.org/10.1002/btpr.2706.
4 Bielser JM, et al. Perfusion Mammalian Cell Culture for Recombinant Protein Manufacturing — A Critical Review. Biotechnol. Adv. 36(4) 2018: 1328–1340; https://doi.org/10.1016/j.biotechadv.2018.04.011.
5 Zeh N, et al. Cell Line Development for Continuous High Cell Density Biomanufacturing: Exploiting Hypoxia for Improved Productivity. Metabolic Eng. Commun. 13, 2021: e00181; https://doi.org/10.1016/j.mec.2021.e00181.
6 Zeh N, et al. Exploring Synthetic Biology for the Development of a Sensor Cell Line for Automated Bioprocess Control. Scientif. Reports 12(1) 2022: 2268; https://doi.org/10.1038/s41598-022-06272-x.
7 Process Parameter Shifting: Part 1. Effect of DOT, pH, and Temperature on the Performance of Epo-Fc Expressing CHO Cells Cultivated in Controlled Batch Bioreactors. Biotechnol. Bioeng. 94(6) 2006: 1033–1044; https://doi.org/10.1002/bit.21013.
8 Li F, et al. Cell Culture Processes for Monoclonal Antibody Production. mAbs 2(5) 2010: 466–479; https://doi.org/10.4161%2Fmabs.2.5.12720.
9 Kwon T, et al. Microfluidic Cell Retention Device for Perfusion of Mammalian Suspension Culture. Scientif. Reports 7(1) 2017: 6703; https://doi.org/10.1038/s41598-017-06949-8.
10 Konstantinov KB, Cooney CL. White Paper on Continuous Bioprocessing May 20–21 2014 Continuous Manufacturing Symposium. J. Pharm. Sci. 104(3) 2015: 813–820; https://doi.org/10.1002/jps.24268.
11 Janoschek S, et al. A Protocol to Transfer a Fed-Batch Platform Process into Semi-Perfusion Mode: The Benefit of Automated Small-Scale Bioreactors Compared to Shake Flasks As Scale-Down Model. Biotechnol. Prog. 35(2) 2019: e2757; https://doi.org/10.1002/btpr.2757.
12 Huang YM, et al. Maximizing Productivity of CHO Cell-Based Fed-Batch Culture Using Chemically Defined Media Conditions and Typical Manufacturing Equipment. Biotechnol. Prog. 26(5) 2010: 1400–1410; https://doi.org/10.1002/btpr.436.
13 Chance B. Reaction of Oxygen with the Respiratory Chain in Cells and Tissues. J. Gen. Physiol. 49(1) 1965: S163–S195; https://doi.org/10.1085/jgp.49.1.163.
14 Hubbi ME, Semenza GL. An Essential Role for Chaperone-Mediated Autophagy in Cell Cycle Progression. Autophagy 11(5) 2015: 850–851; https://doi.org/10.1080/15548627.2015.1037063.
15 Hubbi ME, Semenza GL. Regulation of Cell Proliferation By Hypoxia-Inducible Factors. Am. J. Physiol. Cell Physiol. 309(12) 2015: C775–C782; https://doi.org/10.1152/ajpcell.00279.2015.
16 Scarcelli JJ, et al. Strategic Deployment of CHO Expression Platforms to Deliver Pfizer’s Monoclonal Antibody Portfolio. Biotechnol. Prog. 33(6) 2017: 1463–1467; https://doi.org/10.1002/btpr.2493.
17 Wang B, et al. High-Throughput Screening of Antibody-Expressing CHO Clones Using an Automated Shaken Deep-Well System. Biotechnol. Prog. 34(6) 2018: 1460–1471; https://doi.org/10.1002/btpr.2721.
18 Zhong X, et al. Transient CHO Expression Platform for Robust Antibody Production and Its Enhanced N-Glycan Sialylation on Therapeutic Glycoproteins. Biotechnol. Prog. 35(1) 2019: e2724; https://doi.org/10.1002/btpr.2724.
19 Xu S, et al. Bioreactor Productivity and Media Cost Comparison for Different Intensified Cell Culture Processes. Biotechnol. Prog. 33(4) 2017: 867–878; https://doi.org/10.1002/btpr.2415.
20 Kimura S, Omasa T. Genome Sequence Comparison Between Chinese Hamster Ovary (CHO) DG44 Cells and Mouse Using End Sequences of CHO BAC Clones Based on BAC-FISH Results. Cytotechnol. 70(5) 2018: 1399–1407; https://doi.org/10.1007/s10616-018-0233-5.
21 Chung CY, et al. Combinatorial Genome and Protein Engineering Yields Monoclonal Antibodies with Hypergalactosylation from CHO Cells. Biotechnol. Bioeng. 114(2) 2017: 2848–2856; https://doi.org/10.1002/bit.26375.
22 Laux H, et al. Degradation of Recombinant Proteins By Chinese Hamster Ovary Host Cell Proteases Is Prevented By Matriptase-1 Knockout. Biotechnol. Bioeng. 115(10) 2018: 2530–2540; https://doi.org/10.1002/bit.26731.
23 Škulj M, et al. Reduction in C-Terminal Amidated Species of Recombinant Monoclonal Antibodies By Genetic Modification of CHO Cells. BMC Biotechnol. 14, 2014: 76; https://doi.org/10.1186/1472-6750-14-76.
24 Lin N, et al. Overexpression of Serpinb1 in Chinese Hamster Ovary Cells Increases Recombinant IgG Productivity. J. Biotechnol. 193, 2015: 91–99; https://doi.org/10.1016/j.jbiotec.2014.10.040.
25 Han YK, et al. Bcl-x(L) Overexpression Delays the Onset of Autophagy and Apoptosis in Hyperosmotic Recombinant Chinese Hamster Ovary Cell Cultures. J. Biotechnol. 156(1) 2011: 52–55; https://doi.org/10.1016/j.jbiotec.2011.07.032.
26 Fu T, et al. Regulation of Cell Growth and Apoptosis Through Lactate Dehydrogenase C Over-Expression in Chinese Hamster Ovary Cells. Applied Microbiol. Biotechnol. 100(11) 2016: 5007–5016; https://doi.org/10.1007/s00253-016-7348-4.
27 Lee P, Chandel NS, Simon MC. Cellular Adaptation to Hypoxia Through Hypoxia Inducible Factors and Beyond. Nature Rev. Molec. Cell Bio. 21(5) 2020: 268–283; https://doi.org/10.1038/s41580-020-0227-y.
28 Gonzalez FJ, Xie C, Jiang C. The Role of Hypoxia-Inducible Factors in Metabolic Diseases. Nature Rev. Endocrinol. 15(1) 2018: 21–32; https://doi.org/10.1038/s41574-018-0096-z.
29 Javan B, Shahbazi M. Hypoxia-Inducible Tumour-Specific Promoters As a Dual-Targeting Transcriptional Regulation System for Cancer Gene Therapy. Ecancermedicalscience 11, 2017: 751; https://doi.org/10.3332/ecancer.2017.751.
30 Stockmann C, Fandrey J. Hypoxia-Induced Erythropoietin Production: A Paradigm for Oxygen-Regulated Gene Expression. Clin. Exper. Pharmacol. Physiol. 33(10) 2006: 968–979; https://doi.org/10.1111/j.1440-1681.2006.04474.x.
31 Kimura H, et al. Identification of Hypoxia-Inducible Factor 1 Ancillary Sequence and Its Function in Vascular Endothelial Growth Factor Gene Induction By Hypoxia and Nitric Oxide. J. Biolog. Chem. 276(3) 2001: 2292–2298; https://doi.org/10.1074/jbc.m008398200.
32 Bergeron M, et al. Role of Hypoxia-Inducible Factor-1 in Hypoxia-Induced Ischemic Tolerance in Neonatal Rat Brain. Ann. Neurol. 48(3) 2000: 285–296.
33 Lee JW, et al. Hypoxia Signaling in Human Diseases and Therapeutic Targets. Exper. Molec. Med. 51(6) 2019: 1–13; https://doi.org/10.1038/s12276-019-0235-1.
34 Rathore AS, et al. Bioprocess Control: Current Progress and Future Perspectives. Life 11(6) 2021: 557; https://doi.org/10.3390/life11060557.
35 Lee JH, et al. NFAT5 Induction and Its Role in Hyperosmolar Stressed Human Limbal Epithelial Cells. Invest. Ophthalmol.Vis. Sci. 49(5) 2008: 1827–1835; https://doi.org/10.1167/iovs.07-1142.
36 Chua OWH, et al. Role of Nuclear Factor of Activated T-Cells 5 in Regulating Hypertonic-Mediated Secretin Receptor Expression in Kidney Collecting Duct Cells. Biochim. Biophys. Acta 1859(7) 2016: 922–932; https://doi.org/10.1016/j.bbagrm.2015.12.009.
37 Aramburu J, et al. Regulation of the Hypertonic Stress Response and Other Cellular Functions By the Rel-Like Transcription Factor NFAT5. Biochem. Pharmacol. 72(11) 2006: 1597–1604; https://doi.org/10.1016/j.bcp.2006.07.002.
38 Wurm FM. Production of Recombinant Protein Therapeutics in Cultivated Mammalian Cells. Nature Biotechnol. 22(11) 2004: 1393–1398; https://doi.org/10.1038/nbt1026.
39 Lin H, et al. Principles and Approach to Developing Mammalian Cell Culture Media for High Cell Density Perfusion Process Leveraging Established Fed-Batch Media. Biotechnol. Prog. 33(4) 2017: 891–901; https://doi.org/10.1002/btpr.2472.
40 Shojaosadati SA, et al. Recent Advances in High Cell Density Cultivation for Production of Recombinant Protein. Iran. J. Biotechnol. 6(2) 2008: 63–84; https://www.sid.ir/en/journal/ViewPaper.aspx?ID=127769.
41 Lee SY. High Cell-Density Culture of Escherichia coli. Trends Biotechnol. 14, 1996: 98–105; https://doi.org/10.1016/0167-7799(96)80930-9.
42 Baez A, Shiloach J. Effect of Elevated Oxygen Concentration on Bacteria, Yeasts, and Cells Propagated for Production of Biological Compounds. Microb. Cell Fact. 13(181) 2014: https://doi.org/10.1186/s12934-014-0181-5.
Corresponding author Linus Weiß is a doctoral student, and Kerstin Otte is a professor at the Institute of Applied Biotechnology, Biberach University of Applied Sciences, Karlstraße 11 (Postanschrift), 88400 Biberach, Germany; 49-7351-582-455; [email protected]; www.hochschule-biberach.de. Nikolas Zeh is a postdoctoral researcher in cell-line development (bioprocess development biologicals) at Boehringer Ingelheim GmbH & Co KG in Biberach, Germany.
You May Also Like





