The Industry’s Hesitation to Adopt Continuous Bioprocessing: Recommendations for Deciding What, Where, and When to ImplementThe Industry’s Hesitation to Adopt Continuous Bioprocessing: Recommendations for Deciding What, Where, and When to Implement
April 17, 2017

Figure 1: A guided decision process for continuous bioprocessing (1)
The US Food and Drug Administration has stated its appreciation of continuous bioprocessing (CBP), and some studies have shown that it can save manufacturers time and money. However, the bioprocessing industry is still reluctant to implement continuous bioprocessing right away. It will be interesting to see which companies will be among the first-movers to harness the competitive benefits.
Although few biologics today are made using CBP-enabled equipment (e.g., advanced bioreactors), the industry is changing. For biologics already in production, it is difficult to displace existing processes. But for early stage pipeline biologics, adopting new process technologies can be easier from a regulatory and a manufacturing strategy perspective. The biopharmaceutical industry has not yet fully embraced CBP models. However, it is maturing and expanding globally, which means its willingness to adapt to meet different needs and requirements is changing.
The “Conveyor Belt” of the Biopharmaceutical Industry
After decades of batch production, it might be difficult for some biomanufacturers to visualize the benefits of continuous bioprocessing. Consider the impact that batch-production methods would have on other industries. In the automobile industry, for example, a batch-production method would mean identical cars are produced in one go from start to finish, and only the cars that pass a final quality test would be sold.
Although that concept might work for a small number of luxury cars, it is impractical and costly for large-scale, low-cost production (which makes up most of the automobile industry). Enter the conveyor belt: The key driver for this process was to reduce cost, but it also provided two additional benefits to the automobile industry that also could be relevant for the biopharmaceutical industry.
The first added benefit is the introduction of a higher quality level. A conveyor belt requires good process understanding to be performed successfully. So if a quality or manufacturing issue occurs during production, the conveyor belt stops, halting an entire production and catalyzing the need for a reliable solution.
The second added benefit is increased and twofold flexibility. First, a conveyor belt makes adjusting the production output to market demand relatively easy. Second, having flexibility provides the possibility of making slightly different types of cars (e.g., different colors) based on one platform.
Of course, the biopharmaceutical industry is different from the automobile industry. One differentiator is the latter’s higher focus on manufacturing costs. This is why, in general, the biopharmaceutical industry has a conservative approach to manufacturing solutions and a reluctance to introduce continuous processing. But CBP and other emerging production technologies are more than just trends. They can provide a number of benefits that appeal to both manufacturers and regulatory authorities.

Table 1: Comparison of batch and continuous bioprocessing manufacturing; the key differences (and therefore, the key benefits) of continuous bioprocessing include increased productivity per square meter and improved product quality (1).
Why Continuous Bioprocessing?
The benefits of operating bioprocesses continuously rather than in batch mode include reduced cost, increased productivity, improved quality, and increased flexibility. These advantages are similar and complementary to those of using single-use and modular systems. Facilities and process lines running continuously are generally more cost-effective, with the same or more product manufactured in the same or shorter time period, thus increasing productivity. And CBP can require less infrastructure, fewer utilities, smaller space, reduced investment, and a smaller staff.
What Is Holding Back CBP Adoption?
The issues restricting implementation of CBP are not just a matter of implementation. Difficulties in adopting CBP are surmountable using current technology. Other limiting factors include a lack of practical know-how and precedents, difficulties with coupling cost-effective CBP-specialized equipment, and inherent industry conservatism. Pharmaceutical manufacturing companies have vocalized the following concerns:
Need for precedence (someone needs to get through the regulatory process first)
Need for robust process analytical technology (PAT) tools, a defined regulatory path, and robust single-use technology
Lack of comfort level and control tools
Lack of easy fit for CBP into existing infrastructure, facilities, and quality systems
Need for economic justification and adaptation of current quality or regulatory programs
Need for unit operations to be fully developed for continuous processing (because it is not a standard platform).
Concerns with adopting CBP can best be summarized by two key issues: lack of experience and concern over the authorities’ point of view. Both are rectifiable and addressed herein. Nevertheless, just as CBP concerns can be summarized in two key issues, two significant benefits can be drawn from the many comparisons between CBP and batch production: increased productivity per square meter and overall product quality improvement.
From FDA’s Blog |
|---|
“This year marked another significant step toward integrating into pharmaceutical production. For the first time, the FDA approved a manufacturer’s change in [its] production method from ‘batch’ to continuous manufacturing. This new approval is for manufacturing Janssen Products, LP’s, medication for the treatment of HIV-1 infection, Prezista (Darunavir). “The company’s efforts in manufacturing advancement were facilitated by the use of FDA’s recently released draft guidance for the industry titled Advancement of Emerging Technology Applications to Modernize the Pharmaceutical Manufacturing Base to help manufacturers implement a variety of technological advancements.” |
— Posted 12 April 2016 on FDA Voice, by Lawrence Yu, PhD |
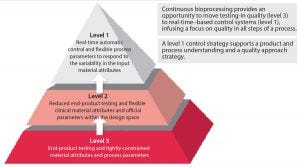
Figure 2: The FDA’s three-tiered control strategy (2)
Deciding What’s Best for You
Changing to the more flexible and agile production method that continuous processing offers is not a trivial mission. Companies need to develop manufacturing processes in a new way, and facilities must be designed differently. I will not outline the requirements of converting to a CBP method, but I pose a few key questions to consider before making the switch. Figure 2 illustrates a structured approach for some of those key questions.
The FDA’s “Stamp of Approval”
Often, regulatory concerns are said to hinder continuous bioprocessing adoption, but the FDA and other agencies are open to this approach. Moreover, regulatory authorities are aware that they need to play an active role in reducing manufacturing costs. That effort began over a decade ago with the 2004 introduction of the FDA’s report on pharmaceutical quality for the 21st century. Moreover, several high-ranking FDA officers — led by Janet Woodcock, director of FDA’s Center for Drug Evaluation and Research (CDER) publicly voiced support to take advantage of continuous processing. That FDA initiative includes an expectation to the industry to establish and demonstrate a higher level of process understanding and a risk-based quality approach, which is a key enabler and requirement for successful implementation of continuous processing.
The FDA has published a presentation of its perspective on continuous manufacturing (CM) (2). The slides highlight the following advantages of implementing a CM approach, all of which can positively influence quality:
Integrated processing with fewer steps (elimination of manual handling, increased safety, shortened processing times, improved efficiency)
Smaller equipment and facilities (operational flexibility, reduced inventory, lowered capital costs, fewer work-in-progress materials, reduced ecological footprint)
On-line monitoring and control for increased product quality assurance in real time (amenable to real-time release-testing approaches and consistent quality).
Enabling an Active Control Strategy
One hindrance to implementing continuous bioprocessing is a lack of experience in the field. Manufacturers face difficulties related to the technical complexities of some systems, which can require more knowledge of processes and on how they must be controlled. Thus PAT and other types of advanced process models for multivariate process control (based on high levels of process and product understanding) are key enablers for successful continuous operation.
Companies starting on this journey need not aim for a 100% continuous facility from the start. Reaching for “low-hanging fruits” and demonstrating the advantage of continuous processing in selected parts of a process reduces skepticism. A hybrid solution often will be the most cost-effective option and can provide the highest chance for a successful outcome while facilitating the maintenance of active control.
In a hybrid solution, an important design decision is selecting which process modules will operate continuously and which will remain in a batch mode (because of their significant impact on the equipment design and space needed for operation). Typically, a batch-mode manufacturing approach requires larger areas for both core process equipment and supporting vessels for solvents, holding tanks, and so forth. That offers the possibility for conversion of some batch processes to continuous operation later when experience with such operations has increased and benefits to cost and flexibility are verified. Naturally, it is advantageous to be proactive during the design and construction phases and include the possibility of an increased use of CBP at a later time in a facility’s lifecycle.
Changes Are Coming Slowly
Basic changes in biomanufacturing paradigms take time — often decades. The biopharmaceutical industry is a highly regulated one, and regulators must be fully convinced that manufacturing changes do not compromise drug quality and patient safety. As we have seen, industry perceptions of problems and risks with CBP often lag behind current implementations. The industry also is conservative. It took over a decade for single-use systems to become dominant for routine precommercial-scale operations, and another decade might pass before such processes are substantially adopted for large commercial-scale manufacturing.
There is no doubt that the industry is transforming. It will fulfill the regulatory authorities’ wishes for the introduction of emerging technologies. And for the benefit of all patients, manufacturers will build on the industry’s supply of high-quality products at an affordable price.
Reference
1 Munk M. Continuous Bioprocessing: What Is Holding the Industry Back from Implementing CBP More Broadly? NNE Pharmaplan, November 2015.
2 Lee S. Regulatory Initiatives for Supporting Innovation in Pharmaceutical Manufacturing. PDA/FDA Joint Regulatory Conference, September 2015.
Morten Munk is global technology partner at NNE, Nybrovej 80, DK-2820 Gentofte, Denmark; 45-3079-2254; [email protected]; www.nne.com.
You May Also Like





