Development Strategies for Novel Vaccines for Infectious DiseasesDevelopment Strategies for Novel Vaccines for Infectious Diseases
October 1, 2013
In a vaccine development program, the probability of success at each transition decreases, even though the actual probability of moving from one phase to another can be 50–80% (Figure 1). Many compounds and vaccine candidates are screened out even before they get into preclinical studies. Developers can implement different approaches to reduce product failure risk before a program gets expensive, including
Establishing a product development plan (PDP)
Identifying and mitigating risk with gap analysis
Learning from the mistakes of others
Investing in expertise (regulatory or chemistry, manufacturing, and controls) as early as possible
Outsourcing with appropriate oversight.

Figure 1: ()
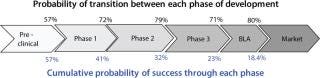
Figure 1: ()
Product Development Planning
Developing vaccines is risky, so prudent companies take efforts to mitigate risk. A robust plan is critical to robust product development. One approach is to begin by envisioning the final vaccine product. A gap analysis is important to identify potential problems. Then you can establish a mitigation strategy to prevent potential difficulties or to discuss different options to reduce that risk.
A robust document should gather everything together in one place (e.g., preclinical data and processes used). That helps you focus resources where they’re needed.
A PDP can be used to define a statement of work for outsourced activities or when you are ready to conduct manufacturing or good laboratory practice (GLP) toxicity studies. A product development plan is a living document. It changes through development and as you obtain more data and better focus through down-selecting vaccine candidates or moving from one phase to the next.
Key decisions must be made at the outset. Common questions my company asks its clients are “What is your focus? Is your focus to get to phase 1 and then license it to Big Pharma, form your own company, or solicit investors? Do you think the product you’re making is the best one and will have legs past a specific phase?” Those questions determine the strategies you will take for each specific case.
A target product profile (TPP) is a useful tool for planning R&D activities throughout development. It allows you to put all characteristics, features, attributes, and everything desired for a marketed product in one place so that you can look beyond each phase or even the marketed product. For example, consider what your product will look like, how it will be administered, its storage requirements, and whether it will need to be stable at room temperature or lyophilized. That will give you a starting point for what you want to see in your vaccine. Then you can work backward and design your development plan to get you there.
Throughout a development pathway are inflection points where you will get information and have to make go–no-go decisions. It’s useful to have a perspective defined so that you can make those decisions. Then, if necessary, you can perform additional work to get to the point where you’re confident that the risk is low going forward. A TPP helps you focus on goals and end results or development efforts that can be used to inform go–no-go decision points.
A TPP should be based on each individual vaccine development program. Some big- and mid-pharma companies prefer to include financial information in their TPP, including market analysis, anticipated costs of goods, sales, and other factors that demonstrate that their vaccine candidates are worth pursuing. You can also develop individual TPPs for different phases.
Figure 2 is a simple TPP example, including a product description stating that it is a vaccine expressing antigen for active immunization against infection with some unknown pathogen and that the mechanism of action is induction of neutralizing antibodies. Everything required to make a product should be listed: formulation, delivery, route, presentation, and the type of vials to be used. In addition, the dose and immunization schedule should be defined along with the targets for storage and shelf life. Clinical information includes primary end points, key claims, and secondary end points.
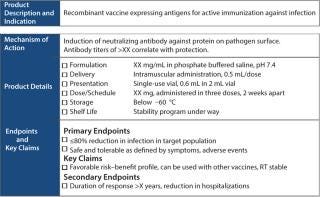
Figure 2: ()
PDP content can be simple or complex. My company tends to make PDPs complex because many clients have never before conducted vaccine development. The content of the plan lays out the background of the disease. If there are known correlative immunities, then they should be listed so that you can evaluate why you think your product should be supported going forward.
At this stage, you should also investigate other vaccines in development (licensed or not) and consider how your product differs from them. Then make a strong case for why yours is superior to existing approaches. The resulting document can be used to solicit funds from funding agencies. It also shows that you’ve thought about many issues that can trip up investigators.
A PDP contains an overview of a product’s history, including how it is made, the source of sequences, virus or bacteria to produce the vaccine, and preclinical immunogenicity (in vitro or in vivo data, neutralization data, challenge data). Make the strongest case for the development of your vaccine that can be made.
You should assess the intellectual property (IP) space and whether other IP claims could infringe or limit your freedom to operate (FTO). The FTO can be extensive or limited, but it behooves you to do at least a rudimentary search at the outset so that you know what else could limit your ability to take it past phase 1.
Finally, gap analysis is an important part of the initial PDP. Once all information is analyzed and synthesized, you can come up with a critical path with go–no-go decision poin
ts and different options to take at each point.
Preclinical Development
An intelligently designed antigen can help prevent some problems in later stages of development, including difficulties during scale-up with solubility, precipitation, and degradation. Such issues can be mitigated at the outset, when your antigen can be changed quickly and inexpensively if necessary. In addition, a range of expression platforms are available, although not all are designed for clinical use. You don’t want to bring novel technologies to the FDA that reviewers have never seen before and for which they may have safety concerns.
The vaccine field has made great strides in even the past five years with respect to cell substrates and other issues. I think that will continue, especially for vaccines that can be highly purified. At early stages, however, you should stay with the easiest possible path toward development. If a particular expression platform is the only system that will make your protein, however, then of course you must use it and justify that to the FDA. But you also have to think about what assays you have to build for host-cell proteins, host-cell DNA, and other factors that the FDA may not have seen before.
Many problems my company addresses are in laboratory-scale process development. Investigators rarely think about scalability of their process steps. Processes used at laboratory scale may not be appropriate for GMP manufacturing and sometimes can be difficult to replace. Preventing such problems when you’re developing a platform simplifies processing.
You should develop as many analytical methods for quality and purity as you can as quickly as possible. You’ll never regret using an analytical method, but you might regret not using one. Early application of orthogonal assays is useful. Many companies will apply a newly developed assay only to later discover a product they wish they had known about earlier when changing directions was less expensive.
Appropriate animal models help define dosage, route, scheduling, and formulation. Preclinical studies provide an early hint about toxicity (the earlier, the better) and help tease out the immune response that you’re looking for. Some animal models such as elderly mice or immunosuppressed mice are specific for different target populations and can help you determine your target population. It all gives you an acceptable risk/benefit ratio and can help elucidate a vaccine’s mechanism of action. The better you understand your product, the more likely it is to move past phase 1.
Preclinical studies are conducted to
recommend an initially safe starting dose and dose regimen in human subjects
identify potential target organs for toxicity related to a product
identify appropriate serological and immunological parameters for monitoring safety and efficacy in human subjects
identify potential “at-risk” populations for administration of the product
help determine an acceptable risk/benefit ratio for human subjects
help elucidate the mechanism of action of a product.
It almost never happens that a material used for proof-of-concept studies and initial in vivo and in vitro studies is truly representative of CGMP material. You’ll have a much different impurity profile when you make CGMP material than when you make laboratory-grade material. You should perform a bridging study or repeat some critical preclinical studies later on. And at a minimum, the required GLP safety study is a repeat-dose study with N + 1 dosing and an accelerated schedule.
Raw materials problems include the lack of a CGMP grade, certificate of analysis, and/or certificate of origin. Using protease inhibitors in a laboratory-scale process is not recommended. In addition, some detergents are not compatible with CGMP. The bioindustry has made some advancement in cell substrates, but if you’re using a novel, tumorigenic cell line, then you might have problems with the FDA. You may need to perform extra testing, which can be extremely expensive.
My advice is to stick to commonly used cell substrates for vaccine manufacturing if at all possible. If you use Chinese hamster ovary (CHO) or NS0 or other rodent cell lines, you will have to perform viral clearance. Everything must be scrutinized for its history of contamination with animal-sourced components (e.g., fetal bovine serum and tryptone). If you can’t trace those in source materials, then you can calculate the risk based on dilutions and the number of passages completed. But it’s better to keep everything clean from the beginning.
Development Issues
Some problems can get in the way of developing a successful product. For example, antigen design, codon optimization, and unpaired cysteines can lead to aggregation and precipitation. If you have a catalytic region in your protein that is an antigen, then you might want to think about ablating that activity and ablate some protease sites to enhance stability.
Inadequate yield is another issue to take into account. What dose will be given to patients? Can your manufacturing platform support that dose at the current yield, even with small improvements that you expect across development? Solubility issues, inappropriate cell substrates, and adventitious agents always should be under consideration.
GMP comes into play when making a master virus seed or a master cell bank. Increasingly, my company is being asked to go back into research laboratories to determine the conditions under which seed materials were made. It would be useful to segregate materials at the laboratory (doesn’t have to be GMP) where the seeds are made. If that isn’t done, then you can go back and do it right before going into GMP manufacture. Rushing to CGMP is a common problem. Some companies will have success in a 1-L flask and think they are ready for a 1,000-L bioreactor, and that’s not often true.
Early Planning Is Key
Product development should be a multidisciplinary approach. Risk mitigation strategy is best implemented at the earliest possible stage. Vaccine development costs hundreds of millions of dollars. And although it can cost tens of thousands to develop a robust product development plan, it is money well spent. A PDP will smooth the transition between the phases and enhance your possibilities of taking your product into the clinic and beyond. A target product profile and PDP are useful tools for planning, risk mitigation, and making the early decisions on a platform and process. Those decisions have long-term implications for timeline, budget, and process risk.
About the Author
Author Details
James Richardson is director of commercial services at ABL, 9800 Medical Center Drive. Building D, Rockville, MD 20850; [email protected].
You May Also Like





