Emerging Technology Trends in Biologics Development: A Contract Development and Manufacturing PerspectiveEmerging Technology Trends in Biologics Development: A Contract Development and Manufacturing Perspective
 For a contract development and manufacturing organization (CDMO), process development and manufacturing of recombinant proteins must be linked because of tight timelines driven by client expectations. Those are in turn driven by a need for rapid progression to clinical testing. Early in process development, the choice of raw materials needs to reflect existing supply chain and manufacturing infrastructure, but remain suitable for scaling up to meet future needs. One approach is to establish platform processes for a class of molecules such as monoclonal antibodies (MAbs). That strategy includes identifying a standard set of raw materials, process parameters, and analytical methods (a control strategy) for the platform manufacturing strategy. Such a platform is well established at Cook Pharmica, where the process development team continues to evaluate new process technologies to assess them for fit and help the company remain flexible in our offerings. Here we share some data generated with an IgG1 at Cook to provide a perspective into some novel process and analytical technologies that enable rapid process development and scale-up to early phase clinical manufacturing.
For a contract development and manufacturing organization (CDMO), process development and manufacturing of recombinant proteins must be linked because of tight timelines driven by client expectations. Those are in turn driven by a need for rapid progression to clinical testing. Early in process development, the choice of raw materials needs to reflect existing supply chain and manufacturing infrastructure, but remain suitable for scaling up to meet future needs. One approach is to establish platform processes for a class of molecules such as monoclonal antibodies (MAbs). That strategy includes identifying a standard set of raw materials, process parameters, and analytical methods (a control strategy) for the platform manufacturing strategy. Such a platform is well established at Cook Pharmica, where the process development team continues to evaluate new process technologies to assess them for fit and help the company remain flexible in our offerings. Here we share some data generated with an IgG1 at Cook to provide a perspective into some novel process and analytical technologies that enable rapid process development and scale-up to early phase clinical manufacturing.
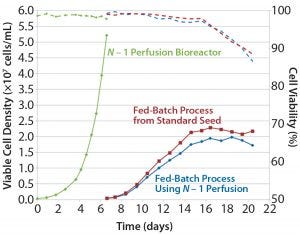
Figure 1a: Comparing platform cell culture process using standard and perfusion N – 1 inoculum — viable cell density and viability
Upstream/Production
Although significant debate continues about integrated continuous biomanufacturing, the near-term application is perfusion for N – 1 seed bioreactors (1, 2). Benefits include overall shorter seed-train durations and an ability to use smaller seed bioreactors, (e.g., 20-L to 50-L scale) to inoculate production bioreactors (2,000-L scale or larger). That provides practical process intensification without significant infrastructure change to existing facility and operations — making it an attractive option for CDMOs.
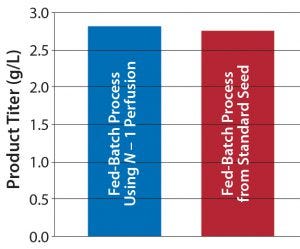
Figure 1b: Comparing platform cell culture process using standard and perfusion N – 1 inoculum — product titer
We evaluated a KrosFlo single-use tangential-flow filtration system from Spectrum Labs. This demonstrated the concept of using a 20-L N – 1 bioreactor to reach a cell density >50 × 106 cells/mL in a six-day perfusion culture. We inoculated two fed-batch production bioreactors, one from the N – 1 perfusion and another from a standard seed culture. Process performance was comparable for both. Figure 1 shows their results for cell density, viability, and product titer. That demonstration shows the possibility for maintaining a standard fed-batch process while shortening its seed train and eliminating large-scale seed bioreactor steps. With continued optimization, this N – 1 perfusion process should be able to reach cell densities close to 100 × 106 cells/mL. This provides more options to optimize clients’ fed-batch production process for shorter durations or increased overall productivity.
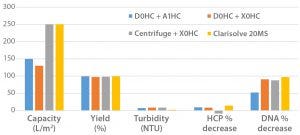
Figure 2: Comparing data from multiple harvest technologies
Downstream Processing
Achieving process intensification in upstream/production increases cell densities and titers, which necessitates debottlenecking of downstream process operations starting with cell culture harvest (3). We designed a study to evaluate different methods of clarifying cell culture harvest from a proprietary fed-batch, platform cell culture process. We evaluated multiple methodologies including centrifugation followed by depth filtration, two-stage depth filtration, and flocculation followed by depth filtration. Flocculation required addition of a flocculating agent — polydiallyldimethylammonium chloride (pDADMAC) from EMD Millipore — followed by single-stage Clarisolve depth filtration system (also from EMD Millipore). That method showed increased capacity along with decreased turbidity, host cell proteins (HCPs), and residual DNA compared with the others (Figure 2).
Later, Cook Pharmica successfully implemented the Clarisolve technology into a client process for a vaccine subunit recombinant protein that had been very problematic for other methods. In this process, traditional depth-filter capacities were greatly reduced (<20 L/m2). Once we implemented a flocculation–Clarisolve harvest step, depth filter capacities increased significantly to about 75 L/m2. This new technology was successfully introduced into phase 1 clinical manufacturing.
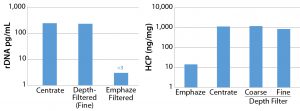
Figure 3: Residual DNA (left) and HCP (right) levels after protein A purification with and without Emphaze hybrid purifier
For another process, we evaluated an Emphaze anion-exchange (AEX) hybrid purifier from 3M as an alternative membrane for harvest clarification in an immunoglobulin G1 (IgG1) antibody purification process (4). According to the supplier, this allsynthetic, charged AEX membrane can provide consistent turbidity of <5 NTU with significant HCP and DNA clearance to improve the performance of protein A capture. In our experience with this membrane in the IgG1 process, we found that product purity after protein A chromatographic purification was at or near injectable quality in terms of HCP and DNA content (Figure 3). HCP levels had been reduced to <10 ppm, and residual DNA content was reduced to <3 ppb. That enabled increased process intensification and performance — particularly for the protein A step.
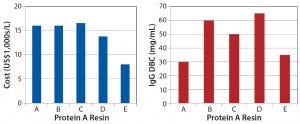
Figure 4: Protein A resin comparison across a growing landscape of multiple suppliers
We have continued evaluations of several high-capacity resins to further reduce downstream bottlenecks for our clients. As an example, we evaluated protein A resins from five different vendors to compare costs and dynamic binding capacities (DBCs) against process performance (Figure 4). All the resins we evaluated provided acceptable step yields for IgG1 near 100% and monomer content near 95% (determined using size-exclusion chromatography) with our platform process. However, we found differences in residual HCP and rDNA (Figure 5), all of which are still acceptable when combined with an overall purification process.
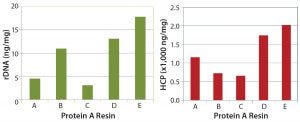
Figure 5: rDNA levels (left) and HCP clearance (right) following protein A capture chromatography
Analytical Technologies
Another way to increase capacity and speed is to use high-throughput analytical methods during screening studies, both for drug-substance processes and drug-product formulation development. When implemented appropriately, these methods are very effective for generating large amounts of useful process data in shortened times. That enables robust processes with rapid turnaround in development — vitally important to CDMO operations — and ultimately shortens technology transfer for good manufacturing practice (GMP) manufacturing. It is especially useful considering the large quantities of samples that can be generated during product/process development. By combining high-throughput methods with automated data analysis, the time for those analyses is also significantly reduced.
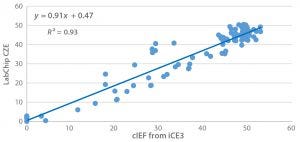
Figure 6: Linear correlation between cIEF and LabChip CEZ for the result of main-peak percentage after seven days of thermal stress (40 °C) on an IgG1 across 96 formulations
For example, we recently implemented LabChip capillary zone electrophoresis (CZE) from Perkin Elmer as a high-throughput analytical method for MAb charge-variant characterization. Figure 6 shows the comparability between capillary isoelectric focusing (cIEF) using a Maurice iCE3 system from ProteinSimple and LabChip CZE for the main-peak percentage of a model IgG 1 MAb. We staged the IgG1 formulated in 96 different formulations on 40 °C storage for seven days, finding very consistent main-peak percentages for both methods, and their results have linear correlation with R2 > 0.9.
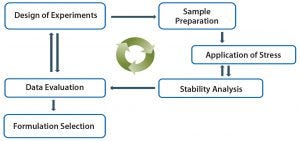
Figure 7: High-throughput formulation development approach
High-throughput analytical methods are particularly important in drug-product formulation development (5). Large numbers of formulations can be screened using a liquid-handling robotic system and 96-well microplates, and samples are analyzed with plate-based high-throughput assays. Typical formulation design is a full-factorial design of experiments (DoE) process with four buffer types, each evaluated at three different pH levels using eight different excipients. Samples are analyzed using high-throughput assays at T0 and after stress (usually thermal stress at 40 °C for one week).
High-throughput assays selected for examining MAb stability require much lower sample volumes and analysis times than conventional analytical methods (6). Such assays can examine thermal, colloidal, physical, and chemical stability of a MAb using
differential-scanning (DSC) fluorometry
a dynamic light-scattering (DLS) plate reader
a spectrophotometer in plate-reader format for absorption at 280 and 350 nm
size-exclusion ultrahigh-performance chromatography (SE-UHPLC)
both reduced and nonreduced capillary electrophoresis with sodium-dodecyl sulfate (CE-SDS) charge-variant assays using a Perkin Elmer LabChip GXII system.
With the above approach, we typically identify top formulation candidates within three weeks of analysis.
Be Nimble, Be Quick
Driving process development innovations and adopting new processes and analytical technologies as appropriate for a given molecule and phase of development are keys to success. CDMOs must remain flexible to meet the growing demands of increasingly complex recombinant protein therapeutics.
Acknowledgments
We acknowledge contributions by Bingqing Wang, Todd Stone, Carl Richey, and all members of the development group in supporting this study. Special thanks to all our suppliers.
References
1 Cooney C, Konstantinov K. White Paper on Continuous Bioprocessing: May 20–21, 2014 Continuous Manufacturing Symposium. J. Pharm. Sci. 21 November 2014; doi:10.1002/jps.24268.
2 Wright B, et al. A Novel Seed-Train Process: Using High-Density Cell Banking, a Disposable Bioreactor, and Perfusion Technologies. BioProcess Int. 13(3) 2015: 16–25.
3 Singh N, et al. Clarification of Recombinant Proteins from High Cell Density Mammalian Cell Culture Systems Using New Improved Depth Filters. Biotechnol. Bioeng. 110(7) 2013: 1964–1972; doi:10.1002/bit.24848.
4 Kester B, et al. Platforming MAb Purification Process Using Matched Component Chromatographic Clarification Approach. 4th International Conference on Accelerating Biopharmaceutical Development: Scottsdale, AZ, 1–4 March 2015.
5 Razinkov VI, Treuheit MJ, Becker GW. Methods of High-Throughput Biophysical Characterization in Biopharmaceutical Development. Curr. Drug Discov. Technol. 10(1) 2013: 59–70.
6 Capelle MA, Gurny R, Arvinte T. High-Throughput Screening of Protein Formulation Stability: Practical Considerations. Eur. J. Pharm. Biopharm. 65(2) 2007: 131–148.
Kumar Dhanasekharan is director of process and analytical development, Kevin Humbard is a senior scientist in cell culture development,Benjamin Kester is a scientist in purification development, Shyamal Choudhari is a scientist in formulation development, Yunsong Li is a principal scientist in formulation and analytical development, and corresponding author Victor Vinci is vice president and chief scientific officer at Cook Pharmica, 1300 South Patterson Drive, Bloomington, IN 47402; 1-812-355-6746; [email protected]; www.cookpharmica.com.
You May Also Like





