Large-Scale AAV Production: Advantages of an Insect-Cell Baculovirus Expression Vector PlatformLarge-Scale AAV Production: Advantages of an Insect-Cell Baculovirus Expression Vector Platform
Adenoassociated viruses (AAVs) have been the center of intensive research since their fortuitous (incidental) discovery in 1965 as a contaminant in a simian adenovirus preparation (1). Initial scientific interest primarily focused on understanding the fundamental biology of this virus type, but later it was harnessed to serve as a genetic vector for use in treating or even curing certain genetic diseases (2). Many distinct characteristics make AAVs a versatile tool for development of clinical candidates, exemplified currently by more than 260 clinical trials in progress for products manufactured using this delivery platform (3).
The safety, broad tropism, and ease of genetic engineering with AAVs are advantageous features of the platform. Despite substantial scientific and industrial interest in the AAV vector, however, large-scale manufacturing remains a major challenge in the field.
To optimize AAV manufacturing, we first must understand the biology of the virus. As indicated by the name of the genus Dependoparvovirus (in the subfamily Parvoviridae) to which AAVs belong, they depend on other viruses (e.g., human adenovirus and herpes simplex virus, HSV) for successful replication (4). So in addition to the AAV genome itself, other genetic elements must be presented (usually transiently) during biomanufacturing to enable production of fully packaged and potent recombinant AAVs. Multiple AAV production platforms have been developed, of which the human embryonic kidney (HEK293) cell line platform with triple plasmid cotransfection has been the most widely used.
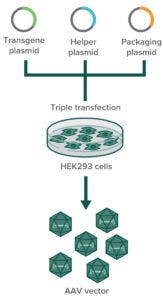
Figure 1: In transfection-based AAV production in HEK293 cells, three plasmids containing the gene of interest (GoI plasmid), the capsid (cap) and replicase (rep) genes (packaging plasmid), and helper-virus genes (helper plasmid) are cotransfected into HEK293 cells. Only those that successfully incorporate all three plasmids can produce AAV particles.
Transfection-Based AAV Production
The HEK293 cell line harbors the early region 1A and 1B (E1A, E1B) genes of adenovirus serotype 5 in its genome, both of which are essential for AAV replication. To produce AAVs, this cell line is transfected with two or three plasmids that together contain the AAV replication and capsid genes (rep and cap, respectively), additional helper (adeno)virus sequences (E2A, E4orf6, and virally associated RNA) (4), and finally the gene of interest (GoI) transgene flanked by AAV’s inverted terminal repeats (ITRs), as depicted in Figure 1. The AAV ITRs are the only sequences that are required to reside adjacent to the transgene for its packaging into a capsid.
The HEK293 production platform relies on transient transfection, which works well at small scale and in adherent-cell cultures. The transfection-based HEK293 system is the most widely used method for AAV production because it provides the fastest and most straightforward way to generate material for a phase 1 clinical trial. HEK293 cells are grown in an adherent-culture format (e.g., in T-flasks) with serum-containing medium for proper growth and cell attachment. At a given target cell density or confluency, transfection is executed through use of a transfection reagent (e.g., calcium phosphate or polyethyleneimine, PEI) and the three plasmids harboring the rep, cap, and GoI sequences. However, this process becomes problematic as a program moves through clinical studies — especially during commercial manufacturing when larger quantities will be required.
The AAV quantity does depend on the therapeutic indication and dosing of a given product. For example, HEK293 is a suitable production system for gene therapies that will be delivered to patients’ eyes: e.g., Luxturna (voretigene neparvovec-rzyl), an approved gene therapy for inherited retinal disease from Spark Therapeutics, has a total dose of 1.5 × 1011 viral genomes (vg). However, for systemic and central nervous system (CNS) applications, including those treating children, HEK293 scalability presents a significant stumbling block. For instance, Zolgensma (onasemnogene abeparvovec) is an approved gene therapy from AveXis/Novartis for treating spinal muscular atrophy (SMA) with a dose of 1.1 × 1014 vg/kg, which is many times higher than that of the Luxturna product.
Traditional laboratory-scale systems for adherent cells generally are difficult to scale up because doing so would require handling large numbers of flasks, roller bottles, or stacked-tray systems during a clinical production run. Not only would that pose a challenge in finding available incubator space at most companies, but the culture and infection of many flasks increases processing time and contamination risks from open manipulation during aseptic processing (e.g., opening and closing the flasks multiple times). Furthermore, producing a typical gene therapy’s clinical dose of 1014 vg/kg for a 70-kg patient would require more than 3,000 T175 flasks of HEK293 culture, assuming a yield of 105 vg/cell and 100% recovery in downstream purification (5).
In response to such concerns, some developers have turned to fixed-bed bioreactors (e.g., Pall’s iCellis system) and suspension-adapted HEK293 cells for improved scalability. Fixed-bed bioreactors allow for adherent growth in a three-dimensional matrix and can be scaled up with changes in bed size. That bioreactor design requires optimization and might not be optimal in all cases, however, because high-density biomass may not provide for effective transfection of all cells (6). Cells grow in small volumes on the fixed-bed matrix, with most of their medium held in an external vessel and continuously recirculated through the compartment that contains the cells. Process parameters such as pH and dissolved oxygen (DO) are controlled in the medium vessel rather than the cell compartment, potentially leading to gradients in those parameters that can affect transfection performance. Additionally, a cell lysis step is required to harvest intracellular AAVs, which can be difficult to perform with a fixed-bed bioreactor.
By contrast, suspension-based cell cultures provide true scalability from laboratory-size culture systems to large, industrial-scale stirred-tank bioreactors without the need for animal serum. Such conditions are preferred for biosafety, consistency, and cost reasons. Compared with fixed-bed bioreactors, suspension-based systems enable easy collection of both cells and culture media.
Efficient transfection of cells in large numbers presents a bottleneck for both adherent and suspension systems with inherent variability, which is not conducive to robust clinical manufacturing. For multiple reasons, triple transfection can be difficult, especially at large scale:
• The high quantities of good manufacturing practice (GMP)–grade plasmid DNA required become a major process-related impurity that is difficult to remove in downstream processing.
• The great quantities of GMP-grade plasmid DNA and transfection reagents needed for large scales are costly.
• Variation in transfection efficiency is high, creating inconsistencies in process outcomes and hence variable product characteristics (6, 7).
• Transfection-based AAV manufacturing seems to be inefficient — only a fraction (5–10%) of cells appear to produce measurable levels of assembled AAV capsids despite high transfection efficiencies (8).
• Helper plasmids often get copackaged into AAV particles that then carry AAV sequences, viral helper sequences, or antibiotic-resistance genes (9). Encapsidation of such sequences into AAVs should be prevented in the interest of in vivo vector safety (helper sequences could be expressed by patients’ cells).
Despite better scalability, and along with production generally of few fully packaged AAVs, the variability of suspension transfection methods continues to impede progress of this product modality.
AAV Production Based on Helper Virus Infection
To address such concerns posed by transfection-based AAV production, some developers are using infection-based systems as an alternative. They repurpose the inherent characteristics of helper viruses for AAV manufacturing (Figure 2). Viruses have evolved to enter cells, and they do so at large scale in suspension as well as they do in nature. When optimal multiplicities of infection (MoIs) are used, all cells are infected, leading to robust production of AAVs — with no additional reagents needed.
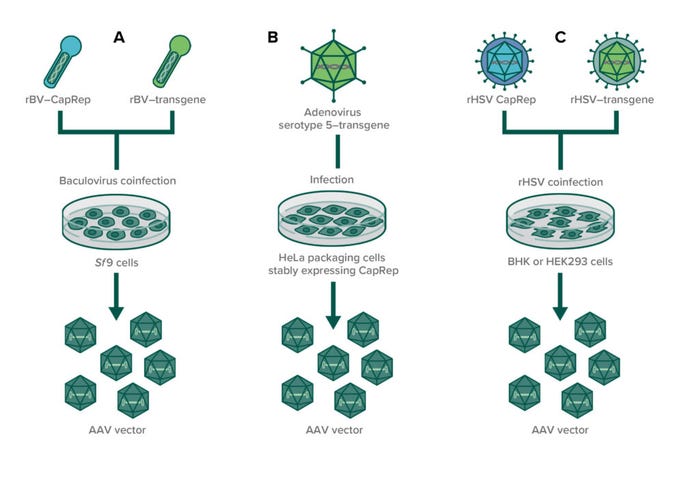
Figure 2: In infection-based production of AAVs through (co)infection, susceptible producer cell lines are infected with recombinant helper viruses to enable AAV production. (A) Two baculoviruses (one expressing CapRep and one expressing the transgene) are used to infect insect cell lines (usually Sf9); (B) an adenovirus expressing the transgene is used to infect HeLa packaging cells that stably express AAV rep and cap genes; (C) two recombinant herpes simplex viruses are used to coinfect either BHK or HEK293 cells to produce AAVs.
A number of helper viruses can provide the necessary factors for AAV replication, and researchers have developed several infection-based systems using recombinant replication-defective viruses to induce AAV production (10). A cell-based manufacturing process using human cervical cancer cells (HeLa cell line) stably expresses AAV rep and cap genes when cells are infected with wild-type adenovirus type 5. The system was established at a 250-L production scale (11, 12). Furthermore, a recombinant HSV-1 expression system has been used in Wave bioreactors from Cytiva, with baby hamster kidney (BHK-21) cells grown in suspension to produce AAV serotypes 1, 2, 5, and 8. That process showed high productivity with up to 1014 vg/L at 10-L scale (13).
The third helper-virus–based system for AAV production is the baculovirus expression vector system (BEVS) used with Lepidopteran insect cells. This system is not new in manufacture of investigational biopharmaceuticals. Baculoviruses have been used extensively for production of several commercially available recombinant proteins, including subunit vaccines and virus-like particles (14–16). Advantages of the system include
• posttranslational modifications that are analogous to those of human cells (insect cells being similarly derived from higher eukaryotes)
• high capacity for transgene insertion in the flexible baculovirus genome
• an improved safety profile because baculoviruses do not infect humans, and adventitious mammalian viruses are unable to replicate in lepidopteran cells
• low probability that residually packaged (baculoviral or host-cell) DNA that could be present in AAV particles will be expressed in patients because no insect or baculoviral genetic elements are actively expressed in mammalian systems (17, 18).
Baculovirus-Based AAV Production
Although the first AAV gene-therapy product on the market, uniQure’s Glybera (alipogene tiparvovec), was made using the baculovirus system, the platform has been optimized primarily for production of therapeutic proteins. A BEVS production system was developed for production of a vaccine containing influenza hemagglutinin to a scale of 21,000 L (19). Compared with HEK293-based methods, baculovirus-mediated AAV manufacturing is less established, with fewer products in the clinic despite its first introduction about two decades ago (20). A single recombinant protein is less complex to produce than a viral vector such as AAV, for which five proteins (VP1, VP2, VP3, Rep78, and Rep52) and a single-stranded DNA molecule must be expressed at optimal levels with correct assembly.
Optimal AAV production requires molecular translation of a mammalian virus (AAV) to insect cells in culture. AAV requires splicing for correct expression of its VP1, VP2, and VP3 capsid proteins and its Rep78 and Rep52 isoforms (21–23). The genetic sequence has evolved to ensure optimal splicing and subsequently correct stoichiometry of AAV’s structural components in mammalian cells. Unfortunately, those processes do not occur optimally in insect cells, and among other things, the naturally occurring viral promoters need to be replaced by promoters that will be active in baculovirus-infected cells. Molecular tinkering thus is required to ensure production of fully packaged and potent AAV particles. Without proper molecular engineering of the viral genes to comply with insect cell expression, the resulting viruses will present incorrect capsid stoichiometry (often the presence of too much VP1), and the percentage of full capsids will be low. The schematic in Figure 3 depicts the different generations of baculovirus production systems so far.
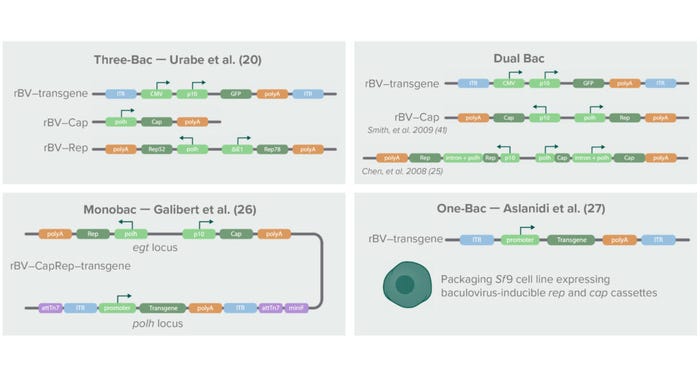
Figure 3: Below is a schematic history of baculovirus-based AAV production systems, the first of which was introduced in 2002 with three baculoviruses containing the transgene of interest, the cap gene, and the rep gene, respectively (20). The cap gene was placed under control of a baculoviral polyhedrin (polH) promoter. The rep isoforms Rep52 and Rep78 were split into two separate expression cassettes under control of a polH pomoter (Rep52) and the Δ immediate early-1 promoter of Orgyia pseudotsugata (OpMNPV) (Rep78). The second-generation system combined cap and rep in a single baculovirus through strategies described by Smith et al. (40) and Chen (25). Later, Galibert et al. developed a Monobac system containing all three elements (cap, rep, and transgene) in a single baculovirus (26). Aslanidi et al. later developed an Sf9-derived packaging cell line that stably expressed cap and rep genes (27). Using those systems for AAV production requires infection with only one baculovirus expressing a transgene.
In the first-generation BEVS, the rep transcripts were split into two cassettes under control of different promoters, and the cap coding sequence was altered to contain a noncanonical start codon (ACG instead of AUG) for proper AAV capsid stoichiometry and genome packaging (20). The genetically altered AAV sequences were cloned into three separate baculovirus constructs harboring either cap, rep, or a transgene flanked by AAV ITRs. For efficient AAV production, a single cell needed to be infected with the three different baculoviruses simultaneously. Although this “Three-Bac” system was successfully applied to production of recombinant AAV serotype 1, it could not be applied to production of other AAV serotypes.
The second-generation BEVS overcame that limitation in AAV serotypes and reduced the number of baculoviruses needed for AAV production to two: a Bac–CapRep virus carrying both the cap and rep genes and a Bac–Transgene carrying the AAV DNA genome (21). Furthermore, different molecular adaptations were introduced to enable proper expression of the AAV genes. For example, Kondratov et al. used an attenuating Kozak sequence and leaky ribosome scanning to achieve correct stoichiometry of the AAV5 and AAV9 capsid proteins (24). That team’s redesigned AAV vectors demonstrated significantly higher biological potencies than their HEK293-manufactured counterparts.
Another strategy for ensuring correct AAV stoichiometry — and thus production of potent vectors with high yields — is insertion of an artificial intron in the AAV sequence. Chen et al. identified a baculovirus-derived intron in which they placed the baculoviral polyhedrin (polh) promoter (25). The artificial intron is introduced into the (AAV) rep and cap genes to enhance transcription and thereby expression levels of VP2/3 and Rep52. The resulting AAVs (of various serotypes) thus produced were infectious and high in titer (up to 1014 vg/L).
Recent developments in the BEVS system include further reduction of the number of recombinant baculoviruses needed for AAV production to only a single recombinant virus. Galibert et al. achieved that by inserting all three AAV genes (rep, cap, and the transgene flanked by AAV ITRs) into one “Monobac” baculovirus (26). Aslanidi et al. generated a baculovirus infection-inducible Spodoptera frugiperda (Sf9) packaging cell line harboring the AAV rep and cap genes in the cellular genome (27). Infection of that packaging cell line with a single baculovirus carrying the transgene flanked with AAV ITRs resulted in production of higher yields of AAV particles than were attainable with the first-generation Three-Bac system.
Without proper molecular engineering of the AAV genes to comply with insect-cell expression, the resulting viruses will have incorrect capsid stoichiometry and a lower percentage of full capsids. In addition, incorrect Rep52 and Rep78 expression timing and levels will lead to packaging of more residual (host and baculoviral) DNA in the AAV capsids. Thus, in characterizing AAV particles from the baculovirus production system, it is always important to understand which specific expression system is used. That greatly influences AAV vector properties such as infectivity, potency, packaging, and manufacturability.
Another challenge associated with BEVS for AAV manufacturing is the inherent genetic instability of the baculovirus vector itself. Baculoviruses naturally excise part of their genomes during viral replication to form mutant viruses, which can become defective interfering particles (28). During subsequent passages of cell culture needed for scale-up, those mutants could outgrow the baculoviruses that have full-length genomes. With recombinant baculoviruses as discussed herein, those expressing (trans)genes could be outcompeted so that AAV production decreases over subsequent passages.
As with improper AAV gene expression and splicing, molecular engineering can overcome baculovirus genetic instability to a large extent. The baculoviral genome can be made more stable through deletion of certain genes or through insertion of transgenes into specific, stable loci in the genome (29, 30). Such strategies have yet to be explored fully for AAV expression. Once the molecular designs of baculoviral vectors and AAV genes are optimized, biomanufacturers will benefit from the many positive features of the BEVS platform for large-scale, robust production of GMP-grade biopharmaceuticals.
Large-Scale AAV Manufacturing
Large-scale manufacturing and commercial production require specific cell-line characteristics such as scalability and high yield. Cells need to grow in low-cost, serum-free media, and the system should be qualified to meet regulatory requirements for purity and safety. Suspension-based production traditionally has taken place in reusable, stainless steel bioreactors (STBRs) up to 50,000 L in capacity or single use STBRs that range from 15-mL to 2,000-L scale. STBRs are highly flexible, offering a broad range of potential operating conditions and efficient gas transfer based on well-understood mixing principles.
Rocking-motion bioreactors also are useful for suspension cultures up to 500-L scale (10). Unlike STBRs, scalability in these systems is limited because they use only headspace aeration, but the systems are easy to operate. Achievable volumes can, for example, be used for medium-scale production, as part of a seed expansion train for large-scale cell-based manufacturing, and for production of (helper) virus seed stocks. Cell substrates in such suspension cultures have shown genetic stability throughout batch productions of several thousand liters (10).
Cell densities reached in STBRs depend on cell type and media but typically range between 5 and 20 × 106 cells/mL for batch and continuous cultures without cell retention. Technologies for perfusion-mode culture available for both STR and rocking systems can increase cell densities through maintaining cells in an optimal metabolic state. For instance, densities of 100 × 106 c/mL have been achieved for Chinese hamster ovary (CHO) cells with on-line biomass measurements and perfusion-rate control (31). To maintain an active biomass, a bioreactor must be equipped with a retention device enabling it to run cultures in perfusion mode.
Such high cell densities help shorten the cell-expansion trajectory before product manufacturing begins. Reaching those densities at an accelerated pace reduces both time and materials needed within a GMP production facility for cell expansion. In addition, the ability to support high cell densities in viral-infection–based production systems creates opportunities for process optimization and intensification based on increasing cell density at infection. If process engineers can maintain cell-specific productivity, then such elevated densities at the time of infection provide for increased volumetric productivity.
Suspension cell lines such as the insect cells and suspension-adapted HEK293 cells can grow with serum-free, chemically defined media in both rocking-motion and stirred-tank reactors, making them both appropriate for large-scale manufacturing and commercial production of AAVs. Merten provided a comprehensive, literature-based comparison of outputs from different upstream AAV production systems and concluded that differences in serotype and vector construct hamper a real comparison — but that, in principle, the different production systems all can achieve AAV titers ranging from 5 × 1013 to 2.4 × 1014 vg/L (32). Using a HEK293 transfection-based process, however, requires large quantities of costly (high-quality, GMP-compliant) plasmids and transfection agents.
In a 2019 cost comparison report, Cameau et al. showed a cost indication (with pricing based on high volume orders of GMP-grade plasmid) of US$100,000 per gram of plasmid DNA (33). In their model, the authors assumed 1.5 µg of plasmid DNA required for transfection of 106 cells for AAV production in HEK293 suspension cultures, requiring investment of $300,000 for plasmid DNA for each 2,000-L production at a transfection density of 106 c/mL. The widely used HEK293-based transient transfection process for viral vector production is easy to perform at laboratory scale, but its challenging process control and variability can present difficulties during scale-up (7).
The BEVS production system already has been used for decades, having evolved from a traditional research tool into a production system for veterinary products and then a manufacturing technology for the production of human therapeutics. Multiple licensed vaccines and therapeutics for human use are produced by insect cells using the system (34). Those include uniQure’s Glybera therapy, which in 2012 was the first gene therapy to receive marketing authorization, as well as a COVID-19 vaccine in 2021. Such a track record proves that if baculoviruses are optimized for genetic stability throughout multiple passages, then true large-scale manufacturing is possible with the BEVS production system.
For example, a cell line originating from S. frugiperda — the expresSF+ cell line from Protein Sciences Corporation — has been used for rapid and efficient scale-up in the Flublok manufacturing process. With it, the company achieved the same process performance at 2-L, 10-L, 100-L, 650-L, and 2,500-L scales, then later scaled up further to a 21,000-L manufacturing process (19, 35). The BEVS uses standardized and fully characterized baculovirus seed stocks for consistent upstream manufacturing. And by contrast with the HEK293 transfection process, infection of Sf9 cells requires no careful mixing control. During manufacturing, baculoviruses carrying the necessary genes to generate a recombinant AAV vector are added into a production bioreactor to infect the Sf9 cell culture. The method minimizes negative impacts to the culture system (from expression of the therapeutic transgene) because the mammalian promoters typically used in AAV vectors show little or no expression in insect cells (18).
Challenges associated with AAV production include low robustness with the production process, high variability of AAV titers, and low/variable percentages of genome-containing particles (7, 36). Upstream process consistency is crucial for enabling a downstream manufacturing process to deliver correct and consistent product quality and yields. Large-scale manufacturing of AAVs must remove downstream techniques that are not efficiently scalable: e.g., centrifugation and gradient ultracentrifugation. Filtration and chromatography techniques are preferred.
One key challenge in downstream processing for AAV manufacturing is to resolve and control the mixture of empty and full capsids, with empty capsids considered to be a process-related impurity. To that end, it is important to produce a full/empty capsid ratio that is as high as possible upstream, thus easing the subsequent removal of empty capsids in the downstream process using ion-exchange chromatography (IEX). It separates particles based on the corresponding difference in their net surface charges, which for AAVs can be positive, neutral, or negative depending on its isoelectric point (pI) and environmental pH. Several chromatography supports can be used, including membrane absorbers (e.g., Pall’s Mustang S type), convective interaction media (CIM from Sartorius BIA Separations) in the form of stationary monoliths, or particle-based adsorbents (Cytiva’s Sepharose resin and Thermo Fisher Scientific’s POROS media).
The pI has been determined for many AAV serotypes, and full AAV capsids have a slightly lower pI than empty ones because of the negatively charged DNA within the capsid structure. Despite that small difference in pI values, IEX can separate them with optimized elution conditions (37). IEX-based processes are reproducible and can be automated and adapted to large-scale operations that fulfill the needs of industrial manufacturing. Substantial development of the IEX method usually is needed, however, because conditions for separating empty and full particles are governed by their physicochemical interplay between the capsid and the encapsidated viral genome.
Regulatory Considerations for AAV Manufacture
Of great interest to gene-therapy developers are accelerated approval pathways. The accelerated-approval paradigm of the US Food and Drug Administration (FDA) can approximate a four-year time frame, rather than the typical 10–11 years for product approval — if the chemistry, manufacturing, and controls (CMC) section of a licensing application is sufficiently robust and if a well-designed early clinical study has demonstrated efficacy (38). For companies wishing to leverage such a paradigm, it is essential to invest in early development of commercial-ready manufacturing platforms that generate robust data for the CMC package as soon as possible using processes that can be scaled up rapidly to commercial production.
Although the BEVS platform requires up-front investment in time and resources to produce and characterize high-quality baculovirus seed stocks, that is balanced by a rapid and linear scale-up trajectory with consistency in both the process and its biological starting materials. Making significant changes to BEVS-based biomanufacturing process during clinical development or large-scale GMP production is a potential strategy that could present significant technical and regulatory challenges and delay programs. Starting with a highly productive, robust, and scalable manufacturing platform to develop a cost-effective manufacturing process will become increasingly important to meeting future therapeutic demands.
Cost of Goods Is Key
The estimated global gene therapy market value is estimated to exceed US$10 billion by 2025 (39). The opportunities offered by such growth have led to a significant increase in the number of companies developing AAV vectors for clinical applications, with more than 250 AAV clinical trials in progress. Demand is increasing for both preclinical and clinical viral-vector manufacturing capacity to support the increasing number of gene therapy development programs. Next-generation AAV production systems will be required to produce sufficient amounts of therapeutic vectors. Each biomanufacturer should choose carefully which production system can cater to its own clinical and commercial needs.
Production platforms based on transfection will provide sufficient yields for orphan-disease indications with relatively low doses (e.g., Luxturna treatment for eye disease). However, as the gene-therapy field transitions to an industry serving larger patient populations with vectors needing systemic administration for an expanding number of disease indications, manufacturing capacity will need to increase by an estimated one to two orders (6).
Once the BEVS has been properly designed at a molecular and process level, the insect-cell system offers clear potential to provide an advanced AAV manufacturing system with favorable cost of goods (CoG). That will help the industry address the rising demand for viral vectors.
References
1 Hoggan MD, Blacklow NR, Rowe WP. Studies of Small DNA Viruses Found in Various Adenovirus Preparations: Physical, Biological, and Immunological Characteristics. Proc. Nat. Acad. Sci. USA 55, 1966: 1467–1474; https://doi.org/10.1073/pnas.55.6.1467.
2 Au HKE, Isalan M, Mielcarek M. Gene Therapy Advances: A Meta-Analysis of AAV Usage in Clinical Settings. Front. Med. 8, 2022: 809118; https://doi.org/10.3389/fmed.2021.809118.
3 Gene Therapy Clinical Trials Worldwide. John Wiley & Sons Ltd.: Oxford, UK, 2022; https://a873679.fmphost.com/fmi/webd/GTCT.
4 Meier AF, Fraefel C, Seyffert M. The Interplay Between Adeno-Associated Virus and Its Helper Viruses. Viruses 12(6) 2020: 662; https://doi.org/10.3390/v12060662.
5 Naso MF, et al. Adeno-Associated Virus (AAV) As a Vector for Gene Therapy. BioDrugs 31, 2017: 317–334; https://doi.org/10.1007/s40259-017-0234-5.
6 van der Loo JC, Wright JF. Progress and Challenges in Viral Vector Manufacturing. Hum. Mol. Gene. 25(R1) 2016: R42–R52; https://doi.org/10.1093/hmg/ddv451.
7 Srivastava A, et al. Manufacturing Challenges and Rational Formulation Development for AAV Viral Vectors. J. Pharm. Sci. 110, 2021: 2609–2624; https://doi.org/10.1016/j.xphs.2021.03.024.
8 Dash S, et al. Only a Small Fraction of Cells Produce Assembled Capsids During Transfection-Based Manufacturing of Adeno-Associated Virus Vectors. Biotechnol. Bioeng. 119(6) 2022: 1685–1690; https://doi.org/10.1002/bit.28068.
9 Chadeuf G, et al. Evidence for Encapsidation of Prokaryotic Sequences During Recombinant Adeno-Associated Virus Production and Their In Vivo Persistence After Vector Delivery. Mol. Ther. 12(4) 2005: 744–753; https://doi.org/10.1016/j.ymthe.2005.06.003.
10 Merten OW, et al. Manufacturing of Viral Vectors for Gene Therapy: Part 1. Upstream Processing. Pharm. Bioprocess. 2, 2014: 183–203; https://doi.org/10.2217/PBP.14.16.
11 Thorne BA, Takeya RK, Peluso RW. Manufacturing Recombinant Adeno-Associated Viral Vectors from Producer Cell Clones. Hum. Gene. Ther. 20, 2009: 707–714; https://doi.org/10.1089/hum.2009.070.
12 Farson D, et al. Development and Characterization of a Cell Line for Large-Scale, Serum-Free Production of Recombinant Adeno-Associated Viral Vectors. J. Gene. Med. 6, 2004: 1369–1381; https://doi.org/10.1002/jgm.622.
13 Thomas DL, et al. Scalable Recombinant Adeno-Associated Virus Production Using Recombinant Herpes Simplex Virus Type 1 Coinfection of Suspension-Adapted Mammalian Cells. Hum. Gene. Ther. 20, 2009: 861–870; https://doi.org/10.1089/hum.2009.004.
14 Patterson RM, Selkirk JK, Merrick BA. Baculovirus and Insect Cell Gene Expression: Review of Baculovirus Biotechnology. Environ. Health Perspect. 103, 1995: 756–759: 3; https://doi.org/10.1289/ehp.95103756.
15 Luckow VA, Summers MD. Trends in the Development of Baculovirus Expression Vectors. Biotechnol. 6, 1988: 47–55; https://www.nature.com/articles/nbt0188-47.
16 Beljelarskaya SN. A Baculovirus Expression System for Insect Cells. Mol. Biol. 36. 2002: 281–92; https://pubmed.ncbi.nlm.nih.gov/19892174.
17 Penaud-Budloo M, et al. Accurate Identification and Quantification of DNA Species By Next-Generation Sequencing in Adeno-Associated Viral Vectors Produced in Insect Cells. Hum. Gene Ther. Meth. 28(3) 2017: 148–162; https://doi.org/10.1089/hgtb.2016.185.
18 Activation of an Insect Baculovirus Promoter in Mammalian Cells By Adenovirus Functions. Dagmar Knebel Virus Res. 8(4) 1987: 317–326; https://doi.org/10.1016/0168-1702(87)90004-9.
19 Cox M. Modern Technology: The Preferred Biosecurity Strategy? Vaccine 35(44) 2017: 5949–5950; https://doi.org/10.1016/j.vaccine.2017.03.048.
20 Urabe M, Ding C, Kotin RM. Insect Cells As a Factory To Produce Adeno-Associated Virus Type 2 Vectors. Hum. Gene Ther. 13(16) 2002: 1935–1943; https://doi.org/10.1089/10430340260355347.
21 Becerra SP, et al. Synthesis of Adeno-Associated Virus Structural Proteins Requires Both Alternative mRNA Splicing and Alternative Initiations from a Single Transcript. J. Virol. 62(8) 1988: 2745–2754; https://doi.org/10.1128/JVI.62.8.2745-2754.1988.
22 Trempe JP, Carter BJ. Alternate mRNA Splicing Is Required for Synthesis of Adeno-Associated Virus VP1 Capsid Protein. J. Virol. 62(9) 1988: 3356–3363; https://doi.org/10.1128/JVI.62.9.3356-3363.
23 Qiu J, Söderlund-Venermo M, Young NS. Human Parvoviruses. Clin. Microbiol. Rev. 30(1) 2017: 43–113; https://doi.org/10.1128/CMR.00040-16.
24 Kondratov O, et al. Direct Head-to-Head Evaluation of Recombinant Adeno-Associated Viral Vectors Manufactured in Human Versus Insect Cells. Mol. Ther. 25(12) 2017: 2661–2675; https://doi.org/10.1016/j.ymthe.2017.08.003.
25 Chen H. Intron Splicing-Mediated Expression of AAV Rep and Cap Genes and Production of AAV Vectors in Insect Cells. Mol. Ther. 16(5) 2008: 924–930; https://doi.org/10.1038/mt.2008.35.
26 Galibert L, et al. Monobac System: A Single Baculovirus for the Production of rAAV. Microorganisms 9(9) 2021: 1799; https://doi.org/10.3390/microorganisms9091799.
27 Aslanidi G, Lamb K, Zolotukhin S. An Inducible System for Highly Efficient Production of Recombinant Adeno-Associated Virus (rAAV) Vectors in Insect Sf9 Cells. Proc. Nat. Acad. Sci. USA 106(13) 2009: 5059–5064; https://doi.org/10.1073/pnas.0810614106.
28 Lee HY, Krell PJ. Generation and Analysis of Defective Genomes of Autographa californica Nuclear Polyhedrosis Virus. J. Virol. 66(7) 1992: 4339–4347; https://doi.org/10.1128/JVI.66.7.4339-4347.
29 Pijlman GP, et al. Evaluation of Baculovirus Expression Vectors with Enhanced Stability in Continuous Cascaded Insect-Cell Bioreactors. Biotechnol. Bioeng. 87(6) 2004: 743–753; https://doi.org/10.1002/bit.20178.
30 Pijlman GP, et al. Relocation of the attTn7 Transgene Insertion Site in Bacmid DNA Enhances Baculovirus Genome Stability and Recombinant Protein Expression in Insect Cells. Viruses 12(12) 2020: 1448; https://doi.org/10.3390/v12121448.
31 Schulze M, et al. Automation of High CHO Cell Density Seed Intensification Via Online Control of the Cell Specific Perfusion Rate and Its Impact on the N-Stage Inoculum Quality. J. Biotechnol. 20(335) 2021: 65–75; https://doi.org/10.1016/j.jbiotec.2021.06.011.
32 Merten OW. AAV Vector Production: State of the Art Developments and Remaining Challenges. Cell Gene. Ther. Ins. 2(5) 2016: 521–551; https://doi.org/10.18609/cgti.2016.067.
33 Cameau E, Pedregal A, Glover C. Cost Modelling Comparison of Adherent Multi-Trays with Suspension and Fixed-Bed Bioreactors for the Manufacturing of Gene Therapy Products. Cell Gene Ther. Ins. 5(11) 2019: 1663–1675; https://doi.org/10.18609/cgti.2019.175.
34 van Oers MM, Pijlman GP, Vlak JM. Thirty Years of Baculovirus-Insect Cell Protein Expression: From Dark Horse to Mainstream Technology. J. Gen. Virol. 96(Pt1) 2015: 6–23; https://doi.org/10.1099/vir.0.067108-0.
35 Buckland B, et al. Technology Transfer and Scale-Up of the Flublok® Recombinant Hemagglutinin (HA) Influenza Vaccine Manufacturing Process. Vaccine 32(42) 2014: 5496-5502; https://doi.org/10.1016/j.vaccine.2014.07.074.
36 Guan J-S, et al. Process Improvement of Adeno-Associated Virus (AAV) Production. Front. Chem. Eng. 4, 2022: 830421; https://doi.org/10.3389/fceng.2022.830421.
37 Dickerson R, et al. Separating Empty and Full Recombinant Adeno-Associated Virus Particles Using Isocratic Anion Exchange Chromatography. Biotechnol. J. 16(1) 2021: e2000015; https://doi.org/10.1002/biot.202000015.
38 Marks P. FDA’s Effort to Advance the Development of Gene Therapy. US Food and Drug Administration: Rockville, MD, 1 May 2019; https://www.fda.gov/news-events/fda-voices/fdas-efforts-advance-development-gene-therapy.
39 Chaudhary G. Gene Therapy Market To Be Worth Over USD 10 Billion by 2025, Predicts Roots Analysis. Business Wire: London, UK, 3 March 2015; https://www.businesswire.com/news/home/20150303005400/en/Gene-Therapy-Market-to-be-Worth-over-USD-10-Billion-by-2025-Predicts-Roots-Analysis.
40 Smith RH, Levy JR, Kotin RM. A Simplified Baculovirus-AAV Expression Vector System Coupled with One-Step Affinity Purification Yields High-Titer rAAV Stocks from Insect Cells. Mol. Ther. 17(11) 2009:1888–1896; https://doi.org/10.1038/mt.2009.128.
Femke Hoeksema is director of process development, Hilde van der Schaar is head of vector generation, Pranav Puri is a senior scientist in bioprocess development, Stefan R. Marsden is a scientist in bioprocess development, Sander van Deventer is chief executive officer, Anthony Newcombe is chief operating officer, and corresponding author Barbara P. Sanders is chief technology officer at VectorY, Science Park, Matrix Innovation Center 408, 1098 XH Amsterdam, The Netherlands; 31-6-343-91-792; [email protected]. Dirk Martens is an associate professor of bioprocess engineering; Gorben P. Pijlman is an associate professor, and Monique M. van Oers is a professor and chair of the laboratory of virology at Wageningen University, Droevendaalsesteeg 1, 6708 PB Wageningen, The Netherlands.
You May Also Like





