Build, Buy . . . or “Rent” Capacity? A New GMP Biomanufacturing Business ModelBuild, Buy . . . or “Rent” Capacity? A New GMP Biomanufacturing Business Model
When it’s time to move from preclinical to clinical-phase product testing, many biopharmaceutical companies face a difficult decision: whether to build or buy a biomanufacturing facility for in-house production or outsource the work to a contract manufacturing organization (CMO). Accinov is a new company that straddles the line between those concepts. Its new biomanufacturing center supports client companies toward clinical development in an innovative way.

NEXITY (WWW.NEXITY.FR)
We propose a groundbreaking model for running good manufacturing practice (GMP) biomanufacturing. Our company’s approach offers a prequalified GMP environment that includes integrated pharmaceutical support services and pharmaceutical responsibilities. This approach allows drug-sponsor companies to run their own processes and to focus on their own core manufacturing activities in a flexible facility that can take them rapidly from preclinical to clinical phase.
Stakes and Challenges
Over the past three decades, biotechnology has introduced dramatic changes in the pharmaceutical industry. Rather than simple A + B chemical reactions, bioprocessing introduces the complexity of working with live microorganisms and cell cultures. And it is moving from stainless steel tanks into plastics and single-use technologies. Meanwhile, regulations have evolved as new technologies have become available over time. Moreover, new entrants to the industry and emerging world markets are strongly intensifying cost pressures on biomanufacturing operations.
It is challenging enough for companies to evolve and keep playing in the game as it progresses so dramatically. Production technologies and the analytical sciences now make available new cell lines and clones within weeks, genome sequencing within days, and analytical results in minutes.
Candidate screening and early process development are difficult during early product phases. The overall development process is becoming fast-paced, and the need for large quantities of biological materials comes quite rapidly in modern project timelines. Biomanufacturing — from culturing bacteria, yeasts, and other eukaryotic cells through downstream processing — is an important investment for small companies moving from preclinical to clinical phases as well as large biopharmaceutical groups needing to optimize capacity and resource use. As more agile and more flexible management of manufacturing becomes required, new models are being proposed.
Strategic Criteria
Uncertainty and risk are definitively major issues faced during the early stages of biological product development. In that context, large capital expenditures are difficult to manage and justify on what can seem like nonproductive investments such as for buildings and infrastructure. Also, industrial operations are strategically prioritized and can be difficult to relocate internally when products reach later stages of development.
Flexibility, cost, and time are therefore key criteria in evaluating options to find the best path forward. Flexibility enables tailored adjustment of biomanufacturing capacity according to evolving needs. That allows companies to release products on time and respond urgently to changes that come in the drug development program as it moves forward.
Financial criteria are critical. No trade-off is acceptable regarding quality attributes and product safety. GMP operations must be conducted in qualified environments by skilled workers. Cost of goods (CoG) and logistics also should be kept in mind. They require specific attention because traceability and controlled materials are part of the GMP regulatory requirements.
The last criterion is time. It is dependent on the other previous parameters because timely delivery of required work packages is a major goal of all drug development programs. Up to a three-year project in itself, construction of a plant is among the most time-consuming aspects of such programs. That includes facility design, construction, and a long qualification process involving regulatory authorities.
Traditional operation models rely on either in-house or outsourced contract manufacturing. However, new models can provide more flexibility and control than either option. Biomanufacturing plants now can be designed in containment and shipped whole to your site or anywhere worldwide. Isolators can be used to complete GMP biomanufacturing in a relatively small footprint in classified areas. In addition to such options, Accinov provides a full solution for manufacturing, including facilities as well as regulatory and quality support to enable GMP bioproduction. This innovative service presents a new alternative model to help companies keep planning and costs under control while retaining process know-how in house.
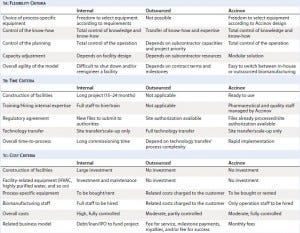
Table 1: Comparing key criteria to enable GMP biomanufacturing of clinical batches
Table 1 highlights the differences between three business models: internal manufacturing, outsourcing, and running operations at a company such as ours. Companies deciding about product manufacturing need to assess certain key criteria: cost structures, capital investments, expertise, and reactivity.
Handling internal capacities could provide a competitive advantage, but designing and maintaining biofacilities require tremendous effort. Such projects are generally difficult to implement at early clinical stages of a development program when capital is dedicated to research and development, preclinical testing, and early clinical studies.
Outsourcing is the preferred option for many small companies and start- ups. The fee-for-service business model of CMOs conveniently supplies clinical materials with a limited demand on internal resources. However, innovative technologies require sometimes long technology transfer processes, and ultimately CMOs share less control of know-how with their clients. So particular attention should be paid to technical agreements and legal contracts that bind such partners to one another.
Our business model presents an alternative solution that allows setting up GMP biomanufacturing with internal resources at early clinical stages, but with no compromise regarding quality attributes and control of the associated know-how. The facility is qualified and ready for use.
The Value of Managing Biomanufacturing Internally
The importance of being involved in chemistry, manufacturing, and control (CMC) operations during product development is often underestimated. Although process development and manufacturing require specific expertise, it is definitely worth the effort to develop that expertise and run operations internally. Unlike outsourcing, this offers faster reactivity in decision making and allows a company to accumulate significant experience — when, for example, identifying process parameters that alter a product’s properties and biological activity. Associated CMC knowledge clearly leads to a better understanding of a product and interpretation of related clinical data.
In addition, few companies consider the value of informal knowledge generated during early R&D phases, which can complicate technology transfer to CMOs. For some highly specific technologies (e.g., particular expression systems), appropriate expertise may be available internally only.
Managing operations internally from R&D through biomanufacturing offers the possibility to fully manage cost and timelines while allowing companies to build the invaluable capital of internal know-how for future projects. From that perspective, our model presents a new solution that provides ready-to-use and GMP-compliant biomanufacturing facilities with integrated support services. Accinov can meet different needs according to a company’s maturity, type of products, or development stage (Table 2):

Table 2: Scenarios and advantages of the solution proposed by Accinov for GMP manufacturing
Small biotech companies must focus on their priorities and comply with regulatory expectations.
Large companies should be careful with their plant capacity regarding current and future needs.
Contract manufacturers can handle project priority diligently to prevent unexpected delays.
Our business model relies on a new 70,000-ft2 (6,500-m2) building designed to meet the high-end requirements of clinical material development and manufacturing (Figure 1). Access to the infrastructure is provided as part of a broader service offering that includes GMP facility management and pharmaceutical support and expertise. Qualified persons and quality- assurance services ensure compliance of activities performed by clients with GMP regulations and the Accinov quality management system.
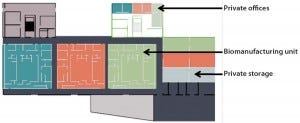
Figure 1: Accinov biomanufacturing facilities
Our quality and pharmaceutical services are responsible for releasing raw materials and drug batches as well as qualifying suppliers and auditing third parties. That allows client companies to focus on their own core bioprocess and analytical activities. Accinov staff members are also dedicated to site management, including technical maintenance and coordination of services such as site security, cleaning, and deliveries. Hosted companies dedicate their own staff to strategic activities without the constraints of fully operating a GMP site on their own.
Our biomanufacturing facilities can enable three different companies to run their operations concurrently in parallel but in complete separation from one another. The facility design and the CGMP site management ensure confidential control of intellectual property (IP) and know- how without risk of cross- contamination.
Pay As You Use
Accinov provides both a GMP environment and pharmaceutical responsibility as a service. First-time players will find a supportive environment to produce their initial GMP batches themselves. Experienced users can quickly operate GMP activities just as they would have done at their own site. Third- party services can be provided without issue because site records can be audited. This new concept offers flexibility for GMP biomanufacturing in a pay-as-you-use model. Our solution presents a real opportunity for hosted companies to create new business models, build new partnerships, and perform agile operations management in support of the current, fast-paced biologics development paradigm. Advantages of this approach include
shorter times to run clinical manufacturing campaigns using owned resources
reduced effort to operate under CGMP compliance
improved product knowledge and strong capitalization on internal know-how and experience
significantly reduced capital expenditures and costs for completing GMP bioproduction.
Accinov is part of the infrastructure offer of Lyonbiopole, a leading biotechnology cluster in southern France. Committed to supporting biotechnology, this cluster has a strong infrastructure policy intended to deliver the most comprehensive support to local companies. Already it manages a business center and the Infectiology Center (http://lyonbiopole.com), a cornerstone of Lyonbiopole’s strategy with biosafety level 2 and 3 research facilities.
Sylvain Peyrache is innovation manager ([email protected]; 33-619- 250-919), and Agnes Nagy is head of business development (agnes.nagy@ accinov.com; 33-629-171-939) at Accinov in Lyon, France; www.accinov.com.
You May Also Like






