Critical Factors for Fill–Finish Manufacturing of BiologicsCritical Factors for Fill–Finish Manufacturing of Biologics
May 17, 2016
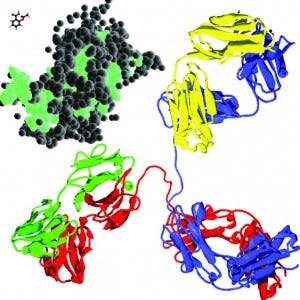
In perspective: aspirin (upper left, front), somatropin (middle molecule), and an antibody (lower right, back) — to approximate scale — molecular models from Wikicommons
Over recent decades, protein-based therapeutics have emerged as key drivers of growth in the pharmaceutical industry. Drug development pipelines have filled with biologics, and a handful of monoclonal antibody (MAb) products have become some of the best-selling drugs around the world. Production of biotherapeutics is often challenging because of the inherent instability of these large, complex molecules. Their fragile nature has forced manufacturers to change how bulk drug substances (BDSs) are handled and final drug product is formulated, sterile filtered, and filled. Ajinomoto Althea was established in 1998 as a contract manufacturer that primarily focused on one class of therapeutics: large-molecule biologics. Here we share some learnings from over 17 years of experience in biomanufacturing and outline special considerations necessary for high-quality biologics fill and finish.
The biologics market is growing 10–15% year over year and now accounts for an estimated 20% of all pharmaceutical sales (1, 2). In 2014, 41 biologics were approved for market in the United States alone, the highest number launched in one year since the 1990s (3). “Big pharma” continues to invest in biologics development programs and manufacturing capabilities (4). Development pipelines are robust, with more than 900 biologic products in development, and all indications point to continued growth of this segment (5).
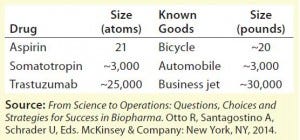
Table 1: Comparing the complexity of different manufacturing processes
Biopharmaceuticals are manufactured by or extracted from living sources. These products are typically proteins, nucleic acids, vaccines, cells, or viruses. In general, when compared with traditional chemically synthesized drugs, biomolecules are larger and more complex, and their quality attributes depend on the biomanufacturing process. One commonly used analogy to help illustrate the complexity of biotherapeutics is to compare their relative sizes with other manufactured goods. Table 1 illustrates that moving from production of small molecules such as aspirin to MAbs such as trastuzumab (Genentech/Roche’s Herceptin), the magnitude of difference is comparable to going from manufacturing bicycles to private jet planes.
Not only are biologics more difficult to produce, with more complex supply chains, but they are also significantly more expensive to develop and manufacture. Because of that demonstrated size as well as the complex physicochemical interactions between a protein and its environment, biologic-based therapeutics must be handled differently from their small molecule counterparts.
Protein Structure and Stability
Protein therapeutics are high– molecular-weight (HMW) polypeptides that require a specific three dimensional structure for their biologically activity. Their structures are considerably more complex than small molecules, consisting of four different structure levels: primary, secondary, tertiary, and quaternary (6, 7).
Primary structure describes the linear sequence of amino acids that make up a protein. Human proteins are made from 20 standard amino acid residues, all differing from one another by specific molecular side chains. A protein’s unique sequence defines its structure and function.
Secondary structure refers to regular local substructures within protein molecules defined by hydrogen bonds between amide hydrogens and carbonyl oxygens. The most common secondary structures are alpha helices and beta sheets.
Tertiary structure describes the overall three-dimensional structure of an entire protein. Its folding is mainly driven by hydrophobic interactions but also stabilized by hydrogen bonds, salt bridges, and disulfide bonds.
Many proteins are made up of multiple polypeptide chains. Quaternary structure refers to how those protein subunits interact with each other and are arranged to form a larger complex. The conformation of protein complexes is stabilized by the same interactions that drive the tertiary structure of their components.
Preventing degradation is essential in maintaining safety and efficacy during the manufacture and long-term storage of a protein therapeutic (7–10). Numerous factors can negatively affect protein stability. A protein’s amino acid backbone (primary structure) can be changed by formation and destruction of covalent bonds (11). Such chemical modifications can be caused by oxidation, deamidation, peptide-bond hydrolysis, disulfidebond exchange, and cross-linking (6, 8–10).
Because the interactions that drive and stabilize higher-order secondary, tertiary, and quaternary structures are inherently weak interactions, proteins are susceptible to physical and conformational degradation. A number of external factors can cause physical degradation: excessively high temperatures, varying acidity or alkalinity, mechanical agitation, high shear forces, and the presence of hydrophobic molecular surfaces, leachables/extractables, nonpolar solvents, and certain excipients (6, 8–10). Proteins are also “sticky” and prone to both adsorption (adherence to surfaces, 10) and aggregation (clumping together, 7–10, 12) because of their hydrophobicity in solution. Aggregates are of special concern because they have been demonstrated to associate with altered biological activity and increased immunogenicity (6, 7, 8, 10).
The potential for protein aggregation can be increased by a number of mechanisms (6, 7, 8, 10, 12–14):
exposure to liquid–air, liquid– solid, and liquid–liquid interfaces
mechanical stresses such as stirring and pumping
freeze–thaw cycles
solution conditions that affect the rate and number of aggregates formed
interactions with metal surfaces
the presence of certain ligands.
At high concentrations, protein–protein interactions will significantly increase the viscosity of a BDS, which in turn decreases its manufacturability and complicates drug delivery (12). Exposure to light can trigger chemical and physical degradation because certain amino acid residues (tryptophan, tyrosine, phenylalanine, and cysteine) are susceptible to photooxidation (8, 9). And in some instances, these factors can work synergistically, one event triggering another mechanism to follow (8). For example, a hydrophobic surface can cause a protein to become misfolded, which can lead to aggregation.
Fill–finish operations must be designed with an awareness of the innate properties of proteins and external factors that can affect a given protein’s behavior and stability (8, 10). Special processes, procedures, and equipment must be in place to ensure product integrity during fill–finish manufacturing.
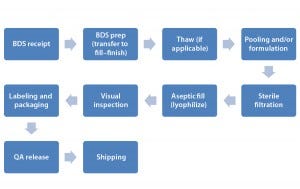
Figure 1: Schematic of drug product (fill–finish) manufacturing
Special Considerations for Fill–Finish of Biologics
Preservation of a protein’s three-dimensional (3D) structure is of vital importance during fill and finish. Because of the many conditions that can negatively affect therapeutic protein structure, function, and stability, very special care must be taken at every step of the biomanufacturing process (6, 7, 8 , 10). Figure 1 illustrates numerous steps and manipulations in biologic drug-product manufacturing.
For biologics manufacturing, special handling procedures must be in place for receipt of an incoming BDS. Quality control (QC) operators are trained in proper BDS handling and visual inspection according to a good manufacturing practice (GMP) specification sheet. Specifications typically cover container integrity, storage conditions (e.g., liquid or frozen), the number of containers, and so on. Once accepted by a QC group, BDS is transferred to an appropriate storage location until its scheduled filling date. Procedures for managing nonconforming biologic BDS also should be in place as part of a manufacturer’s quality system oversight.
End-to-end cold-chain infrastructure and traceability are critical to maintaining the quality of a biologic BDS and drug products (14, 15). Validated storage at 2–8 °C, –20 °C, and –80 °C is sufficient to support most protein biologics. Procedures should include 24-hour temperature monitoring, notification procedures to follow in case of failure, and redundant systems for refrigeration and emergency power. A drug-product manufacturer needs routine preventive maintenance plans to ensure that its equipment is working properly. Intermediate storage for extended hold-times or multiple-day filling processes will require appropriate transfer and storage procedures to maintain sterility and optimal temperature of the BDS.
Freezing a BDS brings a number of advantages in biologics manufacturing: decoupling a BDS from the drugproduct manufacturing process, reducing the risk of microbial contamination, and increasing BDS stability for flexible timelines (8). However, detailed thawing procedures should be in place to ensure that thawing frozen a BDS doesn’t compromise protein activity and stability based on data acquired during product development (13). Once a BDS is thawed, then associated dilution, pooling, and formulation protocols must be gentle to prevent foaming or splashing of the protein solution. Introduction of high shear forces can induce a protein to aggregate or cause conformational changes that affect its activity and/or solubility (11). Thus, low-shear mixing methods are required. Ideally, freezing and thawing steps are both performed in closed systems to prevent exposure of a BDS to outside air, thereby reducing the potential for microbial contamination (16).
Accurate sizing of filters for sterile filtration is important to reduce hold-up and loss of high-value biologic BDS. In both sterile filtration and container–closure filling unit operations, peristaltic pumps have become the standard for most biologics filling processes. Adjusting flow speed to prevent foaming and splashing is simpler with peristaltic than with piston pumps (17). Cleaning and changeover becomes more efficient with such pumps as well because their disposable tubing, connectors, and filling needles are the only materials that directly contact the product (17). And as mentioned above, most biologics are sensitive to shear forces. Peristaltic pumps provide low-pressure pumping for gentler drug product manufacturing (17).
For biologics-based parenteral fill–finish products, manual visual inspection is standard in both the United States and Europe. This QC method relies heavily on the experience, training, and skill of operators (18). Regulators expect a well characterized and robust inspection process. Extensive training is required along with a robust qualification regimen to ensure that operators have the skills necessary to detect a long and thorough list of potential drug-product and container defects (18).
Some challenges facing biologics fill and finish manufacturing operators include
handling of sensitive biologic products
inspection of clear and opaque suspensions
inspection of amber vials
distinguishing between foreign and product-related particulates.
Labeling, packaging, and shipment processes all must adhere to GMP regulations. Proper handling is required to eliminate unnecessary agitation that can adversely affect stability. Throughout preparation and shipping of drug product, the “cold chain” must be maintained. That is, the product must not be subject to temperature fluctuations. Also critical during transport is secure packaging with appropriate protection against temperature fluctuations and mechanical agitation (14, 15).
Temperature monitors, data loggers, and global-positioning system (GPS) trackers also should be included when appropriate. Active, validated shipping containers such as shipping containers from CSafe Global have been proven to transport valuable biologics without compromising product quality.
Other Critical Aspects
Highly concentrated protein solutions can have viscosity levels that present special challenges in drug product fill–finish operations (12, 19). Pumping viscous solutions through small-diameter tubing can generate shearing and other effects that could degrade proteins. Using tubing of different Shore hardness (see “Definitions” box), diameters, and configurations often helps manufacturers handle viscous product solutions. Performing critical unit operations at higher temperatures can reduce viscosity, but a manufacturer must determine the effect of such conditions on a given protein’s stability and activity before choosing that option (19).
Definitions |
|---|
cold chain: a temperature-controlled supply chain that, when unbroken, provides an uninterrupted series of storage and distribution activities to maintain a given temperature range |
cross-link: a bond linking one polymer chain to another (covalent bonds or ionic bonds) |
deamidation: a chemical reaction in which an amide functional group is removed from an organic compound (in proteins, damaging amide-containing side chains of asparagine and glutamine amino acids) |
disulfide-bond exchange: occurs when a thiolate anion (formed by deprotonation of a free thiol), displaces one sulphur of the disulphide bond in an oxidized molecule |
excipients: nondrug substances included in formulations as stabilizers, matrices, fillers, or enhancers |
extractables: chemical species that migrate from plastic materials when exposed to certain solvents under exaggerated temperature and time conditions. |
inoculation: introduction of microorganisms or cells into a culture medium |
leachables: chemical species that migrate from plastics under normal conditions |
ligands: molecules that bind to other (usually larger) molecules |
oxidation: loss of electrons by a molecule, atom, or ion due to interaction with oxygen |
peptide-bond hydrolysis: breakage of a peptide bond through introduction of water |
peristaltic pump: a type of positive-displacement pump that contains fluid within a flexible tube that is fitted inside pump casing |
piston pump: a type of positive-displacement pump with a high-pressure seal that reciprocates with a piston |
shear: a strain in the structure of a substance produced by pressure when layers are laterally shifted in relation to one another |
Shore hardness scale: Defined by Albert Ferdinand Shore in the 1920s, a scale that measures the hardness of polymers, elastomers, and rubbers from D (hard) down to A (medium) and OO (soft) |
Most protein biotherapeutics are made by microbial fermentation or mammalian cell culture. The raw materials, nutrient media, and growth conditions used for such production processes can promote the growth of unwanted microorganisms as well as desirable expression systems (10, 16). Microbial contamination can affect the robustness and repeatability of a biomanufacturing process as well as its final drug product’s purity, potency, and safety (16). Adequate control measures must be in place to prevent microbial growth in biological facilities and equipment (16). They can include a robust cleaning schedule with a set rotation of disinfectant/cleaning agents along with controls that ensure sterility of growth environments before culture inoculation. Low bioburden must be maintained throughout the entire drug-product manufacturing process — from BDS receipt through aseptic filling (16).
Because biologics developers are focusing increasingly on personalized medicine and orphan-drug designations, the industry has seen a significant increase in the number of lower-volume products introduced to market. And many companies outsource their drug-product manufacturing activities even when drug substance is manufactured “in-house.” So most fill–finish operations are performed in multiproduct facilities. Most suchfacilities have converted to single-use technologies at least in part because disposable systems offer many benefits: reducing contamination risk, increasing operational flexibility, and shortening changeover times (20, 21). Efficiencies gained can facilitate and speed up biologics’ movement through clinical trials to commercial launch, therefore increasing a sponsor company’s return on investment.
Maintaining protein stability must take into account many complex physicochemical phenomena that are controlled by both intrinsic and extrinsic factors. Because of the significant number of manipulations necessary for biologics fill and finish, such operations carry the potential risk of disrupting the biologically active state of a therapeutic protein. Formulation, filtration, and filling of drug products is an often overlooked but vital part of biologics development that requires special capabilities to ensure the high quality of products throughout manufacturing, transport, and long-term storage.
Since 1998, Ajinomoto Althea has invested significant resources to build manufacturing platforms that specifically protect the integrity of large-molecule biologics. Clients have come to rely on this experience and expertise to ensure high-quality fill–finish manufacturing of their high-value biotherapeutics.
ADC Considerations |
|---|
In 2015, Althea expanded its existing biological drug-product manufacturing operations to make products such as antibody–drug conjugates (ADCs). Oncology therapeutics including ADCs and highly potent active pharmaceutical ingredients (HPAPIs) represent one of the fasting growing segments in the pharmaceutical industry. These products require specialized manufacturing facilities and infrastructure to ensure their safe handling, manufacture, and delivery. Althea’s new facility will include areas dedicated to bioconjugation, formulation, purification, quality control, and aseptic fill–finish including lyophilization. This 57,000-ft2 plant has been designed for safe handling and manipulation of very low occupational exposure limit (OEL) compounds while maintaining aseptic conditions and GMP compliance. It will accommodate client projects from early clinical phases through commercial launch and supply. |
Commonly Outsourced
The overall biologics market is growing at a 10–15% rate. After analytical testing, drug product fill and finish is the most outsourced activity in biologics, and it is predicted to grow for the foreseeable future. That will translate to steady growth for the outsourced fill–finish market. Contract manufacturing organizations (CMOs) that have the requisite biologics expertise, regulatory track record, and capacity have an opportunity to gain business in this high-growth segment. Since 2003, Althea has been a leading expert in aseptic drug-product filling in both vials and syringes. The company’s experience, expertise, and regulatory track record have brought tremendous revenue growth over the past few years. We anticipate this growth to continue through the coming years.
References
1 Thomas A. Global Biological Drugs Market Will Reach US$287 Billion in 2020. Persistence Market Research: New York, NY, 22 June 2015; www.persistencemarketresearch.com/mediarelease/global-biological-drugs-market.asp.
2 Biologic Therapeutic Drugs: Technologies and Global Markets. BCC Research LLC: Wellesley, MA, January 2015; www.bccresearch.com/market-research/biotechnology/biologic-therapeutic-drugs-technologies-markets-report-bio079c.html.
3 Carroll JD. Biopharma Posts a ChartTopping 41 New Drug Approvals in 2014. Fierce Biotech 2 January 2015; www.fiercebiotech.com/special-reports/biopharma-posts-chart-topping-41-new-drug-approvals-2014/2015-01-02.
4 Van Arnum P. Big Pharma’s Investment in Biologics. PharmTech.com 3 May 2013; www.pharmtech.com/big-pharmas-investment-biologics.
5 Medicines in Development: Biologics. Pharmaceutical Research and Manufacturers Association: Washington, DC, 2013; www.phrma.org/sites/default/files/pdf/biologics2013.pdf.
6 Technical Brief Volume 8. Protein Structure. Particle Sciences Drug Development Services: Bethlehem, PA, 2009; www.particlesciences.com/news/technical-briefs/2009/protein-structure.html.
7 Watts A. Biological Drugs: Practical Considerations for Handling and Storage. University of Bath Department of Pharmacy and Pharmacology: Bath, UK, 22 May 2013; www.slideshare.net/bathasu/biological-drugs-practical-considerations-for-handling-and-storage.
8 Patel J, et al. Stability Considerations for Biopharmaceuticals, Part 1: Overview of Protein and Peptide Degradation Pathways. BioProcess Int. 9(1) 2011: 20–31; www.bioprocessintl.com/manufacturing/formulation/stability-considerations-for-biopharmaceuticals-part-1-332821.
9 Kerwin BA, Remmele RL. Protect from Light: Photodegradation and Protein Biologics. J. Pharmaceut. Sci. 96(6) 2007:1468–1479; doi:10.1002/jps.20815.
10 Kashi R. Challenges in the Development of Stable Protein Formulations for Lung Delivery. AAPS Symposium (Baltimore, MD, 9 September 2011); http://docplayer.net/11725148-Challenges-in-the-development-of-stable-protein-formulations-for-lung-delivery.html.
11 Maa YF, Hsu CC. Effect of High Shear on Proteins. Biotechnol. Bioeng. 51(4) 1996: 458–465; www.ncbi.nlm.nih.gov/pubmed/18629798.
12 Child J. Minireview: Protein Interactions (honors thesis). University of New Hampshire Scholars’ Repository: Durham, NH, Fall 2012; http://scholars.unh.edu/cgi/viewcontent.cgi?article=1081&context=honors.
13 Puri M, et al. Evaluating Freeze–Thaw Processes in Biopharmaceutical Development: Small-Scale Study Designs. BioProcess Int. 13(1) 2015: 34–45; www.bioprocessintl.com/manufacturing/fill-finish/evaluating-freeze-thaw-processes-biopharmaceutical-development-small-scale-study-designs.
14 Butschli J. Assessing Trends in Temperature-Sensitive Biologic Shipments. Life Sciences Logistics 31 August 2015; www.lifescienceslogistics.com/logistics/temp-control-packaging/assessing-trends-temperature-sensitive-biologic-shipments.
15 Burgess B. Packaging Trends for Biologics. Healthcare Packaging 9 April 2013; www.healthcarepackaging.com/applications/healthcare/packaging-trends-biologics.
16 Lolas A. Microbial Control Strategies in Bioprocessing Falling Short of Assuring Product Quality and Satisfying Regulatory Expectations. Am. Pharmaceut. Rev. 2 April 2013; www.americanpharmaceuticalreview.com/Featured-Articles/134040-Microbial-Control-Strategies-in-Bioprocessing-Falling-Short-of-Assuring-Product-Quality-and-Satisfying-Regulatory-Expectations.
17 Lambert P. Dispensing Biopharmaceuticals with Piston and Peristaltic Pumps. PharmTech.com 17 September 2008; www.pharmtech.com/dispensing-biopharmaceuticals-piston-and-peristaltic-pumps.
18 Forcinio H. Trends and Best Practices in Visual Inspection. PharmTech.com 2 March 2014; www.pharmtech.com/trends-and-best-practices-visual-inspection.
19 Palm T, et al. The Importance of the Concentration-Temperature-Viscosity Relationship for the Development of Biologics. BioProcess Int. 13(3) 2015: 32–34.
20 Langer ES, et al. 12th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production. BioPlan Associates, Inc.: Rockville, MD, April 2015; http://bioplanassociates.com/publications/12th_Biomfg_Table_of_Content.pdf.
21 Chao S-B. Biomanufacturing Vision for the Future. June 2013; NIPTE/FDA Research Conference: Future of Pharmaceutical Manufacturing (Rockville, MD, 18–19 June 2013). www.nipte.org/sites/default/files//documents/Biomanufacturing Vision for the Future – Shou-Bai Chao(1).pdf.
Don Paul Kovarcik is a technical marketing specialist at Ajinomoto Althea, Inc., 11040 Roselle Street, San Diego, CA 92121; 1-858-882-0123, fax 1-858-882-0133; [email protected].
You May Also Like





