Cell Harvesting of Biotechnological Processes By Depth FiltrationCell Harvesting of Biotechnological Processes By Depth Filtration

In the pharmaceutical industry, there is a strong desire to introduce new drugs on the market as fast as possible (time-to-market) due to time-limited patent protection for new pharmaceutical products. The thinking behind this desire is simple: the sooner a new product can be introduced to the market, the sooner patients can benefit, and the better are the manufacturer’s prospects of financial returns. This consideration, however, should not allow compromises to enter into the manufacturing process design. This article provides an example of how good process design can both optimize and expedite the harvesting of cells in a biotechnological process.
In practice, there is a tendency to overlook the fact that a purification process developed at a small laboratory scale will not always work smoothly at pilot and commercial process scale. An effective scale-up cannot be taken for granted. Scale-up requires detailed knowledge of the process and of the tools required to implement and control it. The scale-up of primary purification in cell culture technology requires an in-depth understanding of the possibilities and limitations of modern depth filter platforms. The careful and considered development of new biopharmaceutical production processes holds great potential for profitability increases. The early stages of process development pave the way for efficient and cost-effective production.
Variability in Biological Processes
All biological processes are subject to inherent variability. For example, in cell cultures, turbidity and viability (the percentage of living cells with respect to the total number of living and dead cells) may exhibit significant fluctuations. The scale-up of efficient purification methods from laboratory to production in biotechnology therefore presents a particular challenge. The possibility that the performance data of the purification tools used may also fluctuate merely amplifies the problems. In practice, difficulties of this nature can occur with conventional cellulose-based depth filters, which have become firmly established for harvest clarification in recent years.
Up until now, pilot and commercial process scale has required considerably larger filter areas to compensate for these fluctuations and uncertainties than would be needed based on a linear scale-up of laboratory conditions. This approach does not satisfy the requirements for high process efficiency. It merely serves to illustrate how optimal process design can offer considerable cost savings potential.
System Design for Depth Filters
A common problem associated with conventional depth filter modules is that the filter sheets often come into contact with one another nearest the center core of the module due to the housing spindle and compression of the tubular core. This creates a flow resistance gradient which makes the applied differential pressure in the system insufficient for full use of the filter area, thereby preventing reliable scale-up.
A well-conceived improved system design and appropriate mode of operation for depth filters can significantly improve the scalability in cell harvesting of biotech processes. Three key aspects that need to be met by an improved filter platform to qualify it for use from laboratory scale to pilot and commercial process scale are (1) equal use of the entire filter area, (2) identical flow paths, and (3) identical flow resistances.
The Pall Supradisc™ II depth filter module was designed to perfectly meet these requirements. Based on the use of stabilizing plastic components, the Supradisc module design eliminates contact between filter sheets, even with large-area filter modules, and ensures that the entire filter area is equally and fully available. The flow paths and flow resistances are identical from one filter sheet to the next, ensuring identical flow conditions.
The extremely robust cage structure of the Supradisc II module design provides high mechanical stability even under adverse process conditions and makes the entire depth filter module equally available to the flow, as described above. This design concept has been successfully implemented both in small, encapsulated filters for laboratory scale and in large modules and capsules for pilot and commercial process scale.
Operating Modes for Depth Filters
The flexibility to adjust the operating mode of depth filters to meet process requirements also has a significant impact on accurate scalability. What does this mean? The decision whether to use depth filters in a classic T-style filter housing configuration, where the inlet and outlet are located at the same level, or an inline configuration, where the flow goes from the bottom to the top, should not be left to chance. It is indeed true that no measurable differences in filter performance can be detected with small area filters (e.g., Supracap™ 100 capsules), whose mode of operation is largely dependent on the existing pipe work. However, this is not the case with larger systems.
The new Stax™ single-use depth filter capsule provides the first opportunity to use this observation effectively to increase filtration performance at process scale. As designed, the system is capable of operating either in a bottom-in–bottom-out mode (T-configuration) or in a bottom-in–top-out mode (inline configuration). This versatile processing option is facilitated by two different manifold kits (Figure 1).
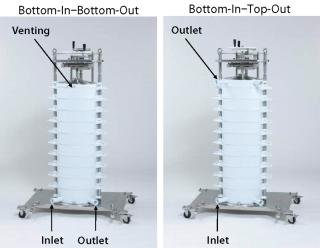
Figure 1: ()
Whether bottom-in–bottom-out or bottom-in–top-out, the flow path through the system is affected by two different phenomena. Firstly, the fluid must overcome a mechanical resistance in the form of dynamic pressure differences, which is identical at each point in the capsules due to the Supradisc II module design, as described above, within each capsule. Secondly, the hydrostatic pressure differences generate a resistance dependent on the system height, which can be up to 150 mbar (2.2 psig) when the system is fully loaded with 10 large capsules.
These hydrostatic pressure differences can be equalized by keeping the fluid level on the upstream and downstream side consistently at the same level. This is achieved by operating in the bottom-in–top-out mode and offers significant advantages over the (classic) bottom-in–bottom-out mode. One such advantage is a considerably more balanced pressure profile which greatly benefits scalability (Figure 2).
on:none;background-color: #f7f7f7;color:#000000;” >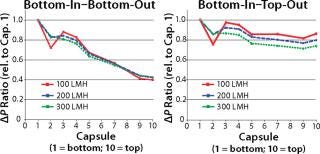

Figure 2: ()
Excellent Scalability
A scalability study compared the filtration performance of a small Supracap 100 capsule filter (0.1 m2 filter area), a conventional Supradisc II module (5 m2) designed for use in stainless steel housings, and several Stax single-use depth filter capsules of varying filter area sizes (2 m2, 6 m2, and 20 m2). While the small area Stax systems (2 m2 and 6 m2) were operated exclusively in bottom-in–bottom-out mode due to negligible hydrostatic pressure differences, the larger area Stax system (20 m2) was used in both the bottom-in–bottom-out and the bottom-in–top-out modes for comparison.
The study confirmed that hydrostatic pressure differences have a major impact on the quality of depth filter system scalability. Nonoptimal operation of large-area depth filter systems caused deviations in scalability of >15% (Figure 3). The bottom-in–top-out configuration is the recommended mode of operation for filter areas of 5 m2 or more (for double-layer depth filter sheets) and 10 m2 or more (for single-layer depth filter sheets). The new Stax single-use depth filter platform combines the advantages of the established Supradisc II module design with the versatility of flexible operating modes that can be arranged to accommodate the given process parameters.
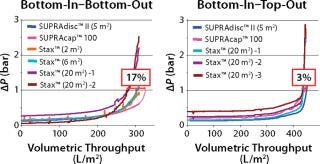
Figure 3: ()
Combined with cross-batch consistency in the production of depth filters (capsules, modules, and sheets) achieved by continuous production processes, this outstanding scalability sets the foundations for the rapid and effective development of economical processes in biotech production.
Summary
Reproducible and reliable scalability of depth filtration for cell harvesting in biotechnological processes depends on both the system design and the operating mode of the depth filters used. With the Stax depth filtration system, Pall has developed a platform that can meet the sophisticated demands of modern cell harvesting processes. Flexible process operating modes, intuitive design and above all, reliable scalability are the key features of this new product range.
About the Author
Author Details
Dr. Manfred Mühl is business development manager of Pall Filtersystems GmbH, Planiger Straße 137, 55543 Bad Kreuznach; 49-671-291-642, fax 49-671-291-643; [email protected].Dr. Dirk Sievers is biopharmaceuticals marketing manager for Pall GmbH Life Sciences, Philipp-Reis-Straße 6, 63303 Dreieich; 49-6103-307-582, fax 49-6103-307-295; [email protected]; www.pall.com.
You May Also Like






