Formulation Development of Microbiome Therapeutics Localized in the IntestinesFormulation Development of Microbiome Therapeutics Localized in the Intestines
Illustration of Clostridium difficile spore-forming bacteria, which cause pseudomembraneous colitis and are associated with nosocomial antibiotic resistance. Kateryna Kon (www.istock.adobe.com)
Scientists have discovered that microorganisms associated with the body play a critical role in human health (1). The intestinal microbiome is the collection of all combined genetic material of microorganisms in the intestines. An intestinal microbiota, a term frequently used interchangeably with microbiome, is the collection of such microorganisms in the intestine. With the volume of research into the human microbiome expanding, so too is the number of examples of its benefits to our health (1). Like diseases of the human genome and physiology, those related to the microbiome are starting to be investigated to their root causes. That has generated new interest in treating microorganisms or metabolites in the microbiome as druggable targets.
A small number of therapeutics target the microbiome, including small- and large-molecule drugs and microorganisms. This number is expected to grow as research continues to illuminate new targets for medicines. Table 1 (below) lists several examples of formulations that are targeting the gut microbiome effectively. Some therapeutics (e.g., acetohydroxamic acid) are inherently resistant to the degradative processes of the gastrointestinal (GI) system (2). However, most therapeutics are modified, inactivated, or catabolized by the environment of the stomach and intestines.
The gut microbiome also can be adversely affected through unintended interactions with treatment modalities targeting disease states in other parts of the body. For example, using antibiotics to eradicate infections disrupts the essential role of many commensal bacteria (3). That approach has led to secondary sequalae (including Clostridium difficile infections) and other diseases involving opportunistic pathogens (4), thus requiring the need to protect a healthy microbiome.
Typically, drug manufacturers develop their products for systemic absorption to elicit desired effects. By contrast, drug manufacturers that develop therapies for delivery into the intestinal microbiome generally strive to restrict systemic absorption and maintain therapeutic effects within the lumen of the intestines. These two diametrically opposed strategies require different considerations for effective drug delivery. Herein we describe the strategies and product attributes to consider for targeted oral delivery of therapies to the gut microbiome and review the approaches in formulation development using examples of two solid oral drug products in development to prevent or treat intestinal diseases.
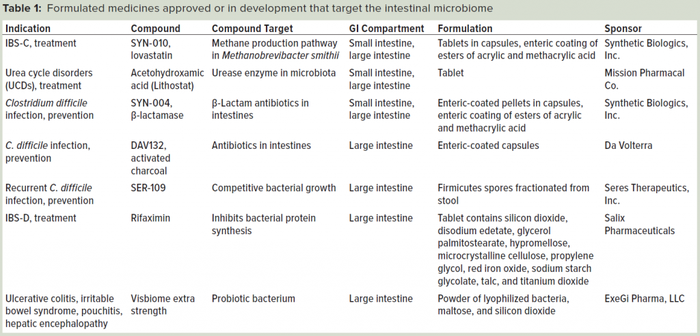
Table 1: Formulated medicines approved or in development that target the intestinal microbiome.
Targeted Microbiome Drug Delivery
Developing therapeutics to be active toward the intestinal microbiome requires delivering them in an active form to the contents and lining of the intestines. Administering medicines to the intestines has its own significant set of challenges. The intestines are designed to catabolize most materials into basic building blocks that can be absorbed readily and used by the body. Those chemical and enzymatic processes can modify many active pharmaceutical ingredients (APIs). Considerations of the variable flow of a complex biochemical mixture through the intestines must be appreciated when approximating the drug’s residence time and stability.
Decisions regarding the formulation of APIs that need to be active in the intestines are based on multifactorial considerations. The final drug product formulation ultimately will be a compromise based on, for example, the chemical and physical characteristics of the API and excipients, their interactions, manufacturing equipment and technologies, patient population, and therapeutic indications. Synthetic Biologics faced many formulation challenges with two of its pipeline products: SYN-004 and SYN-010. The APIs of both products were susceptible to degradation and absorption in the stomach.
For the SYN-010 product, recent studies have implicated the production of methane in the intestines by the archaea Methanobrevibacter smithii in patients with irritable bowel syndrome with constipation (IBS-C). Experimental evidence indicates that methane gas slows intestinal stool transit (5, 6), and high breath methane correlates with IBS-C (5, 7). Studies have demonstrated that lovastatin lactone can inhibit the generation of methane (8). Thus, lovastatin lactone was hypothesized to be a new treatment for IBS-C with a novel mechanism of action that treats the cause of IBS-C rather than the symptoms of the condition.
The goal for SYN-010 product was to colocate lovastatin with M. smithii in the small and large intestines while minimizing the conversion of lovastatin to β-hydroxyacid (HA). Because this conversion can occur anywhere in the GI system, SYN-010 was designed to release a limited amount (7 mg) of lovastatin in the small intestine, and a majority (35 mg) in the large intestine (9).
SYN-004 is a formulated, large-molecule, β-lactamase product. The administration strategy for this enzyme was considerably different from that of SYN-010. β-lactam antibiotic usage can cause dysbiosis of the intestinal microbiome, which has been associated with many health problems, including C. difficile infections (4). Antibiotics disrupt the intestinal microbiome and allow C. difficile to proliferate and produce toxins that can lead to serious illness and death (10).
In addition to their renal excretion, antibiotics are excreted into the small intestine through the hepatobiliary route. The SYN-004 product was formulated as a multiparticulate, designed to begin dissolving upon entering the duodenum to inactivate susceptible intravenous β-lactam antibiotics to prevent disruption of the intestinal microbiome (11). The SYN-004 β-lactamase works by catalyzing the hydrolysis of the lactam ring of susceptible β-lactam antibiotics, such as ceftriaxone and piperacillin (4), thus preventing antibiotic-associated intestinal dysbiosis.
Formulation Considerations
The dosage format is one of the first considerations for developing a new oral formulation designed to remain localized to the intestines. Formulation development technologies and manufacturing machinery can produce a wide range of dosage forms, including tablets, powders, granules, pellets, syrups, nanoparticles, and so on. Dosage forms can be created through a liquid phase, or a number of dry components can be mixed together and directly compressed. The resulting products can be administered “as is” or be further encapsulated for convenience of administration. To provide sustained drug delivery to the intestines, a drug should be dispersed in the stomach and dosed into the intestines as stomach contents are emptied into the duodenum. In such cases, multiparticulate formulations should be considered. To deliver medicines into the matrix present (chyme in the small intestine, feces in the large intestines), the formulation should diffuse well throughout the semisolid to solid matrices.
An important criterion for developing a medicine for the microbiome is protection from the gastric environment, with subsequent targeted release of an API. Excipient selection is critical to achieve dissolution and release to a targeted area (e.g., jejunum, ileum, proximal large intestine, distal large intestine). One physiological characteristic of the GI tract that can be exploited for drug delivery is the pH gradient that ranges from about pH 1 in the stomach to pH 5 in the duodenum, pH 6 in the ileum, and pH 6.5–7.5 in the colon (12).
Many polymers can provide gastric protection of APIs and dissolve and release APIs under certain conditions (13). For example, excipients for enteric protection based on methacrylic acid polymers (e.g., Eudragit L30-D55 copoloymer, Evonik) can be used to coat a drug formulation and dissolve at pH 5.5, releasing APIs in the duodenum (14). Other excipients provide enteric protection — for example, by dissolving under changes in osmotic pressure that occur during transit through the intestines (15) or being enzymatically digested by chitinase produced by bacteria in the large intestine (16).
Once a desired solid oral dosage form is chosen (e.g., pellet, tablet, or capsule), several excipients are selected for mixing experiments with an API. The compatibility of an API with excipients typically is determined using analytical methods known for the API. An unacceptable level of degradation products or loss of activity would exclude certain excipients from further evaluation. Short-term stability studies also are conducted at accelerated conditions (e.g., at 40 °C and 75% relative humidity) to determine excipient compatibility.
A desired release profile can be achieved by using additional excipients. For example, disintegrants can be combined with a formulation to accelerate dissolution of a solid formulation and release of an API (17). Other aids to manufacturing
(e.g., glidants and antitacking agents) can be considered if additional functionality is required (18). Magnesium stearate is used routinely as a lubricant to aid in the machinability of powders used in direct-compression processes (19). The total composition must be tested for compatibility and stability effects with the formulation API.
Many other factors should be considered during development of oral drug products that target an organism or metabolite resident in the intestinal tract. Although these considerations cannot be fully explored here, they include solubility of an API in the environment that it is intended to be active, stability of an API and whether it can be controlled by design improvements, and the manufacturability of a solid dose formulation (e.g., tolerance to high temperatures, compression, and low water content). Such aspects of formulation development also are critical and must be considered as product development proceeds and analytical results are evaluated.
The possible effects of microbiota cytochrome P450 (CYP) isozymes on the metabolism of drug products targeting the microbiome should not be overlooked. Human CYPs are active in modifying or degrading drug products that are systemically absorbed (20). Intestinal microbiota also express numerous CYP isozymes that can modify or degrade drug products in the targeted intestinal region (21–23). The effects of microbiota-derived CYPs could result in decrease or loss of functionality of an oral drug product, and such CYPs should be tested using the appropriate matrix, such as chyme or feces. One approach involves conducting in vitro stability studies using purified CYP isozymes
(e.g., CYP3A or CYP2C9) with a therapeutic agent.
SYN-004 Product Development
The oral dosage form of delayed-release SYN-004 is a capsule that contains the SYN-004 product spray-dried onto sugar cores, which are further spray-dried with a protective enteric polymer (11). The dosage form is designed so that the capsule dissolves in the stomach, releasing gastric-protected pellets that enter into the duodenum, where pH is >5.5. At that pH level, the polymer begins to dissolve and releases the SYN-004 enzyme. The methacrylic acid copolymers are used frequently in solid oral dosage products that require gastric protection (24).
The methodology for developing a drug-layering solution for a coated sugar-core formulation focused on casted film studies to test numerous common excipients (11). The casted film droplet tests were intended to be a high-throughput predictive tool for estimating the physical compatibility among different binder solutions and the SYN-004 product and for accomplishing the highest potential drug loading. With this approach, development time was shorter than that required by using the traditional methodology of drug layering multiple combinations onto sugar pellets. Each droplet test could be conducted in minutes; by contrast, spray-coating trials require hours and expend considerably greater quantities of the drug substance. Altogether, about 125 droplet tests were performed. The outcome of that droplet screening provided a framework of different formulations, with qualitative assessments that reinforced decision making (11).
In view of those droplet-test results, the study showed that the SYN-004 solution containing buffer salts was prone to cracking (11). Compatible binding solutions were necessary to overcome this propensity. Hydroxypropyl cellulose (HPC, Klucel EF brand) was the most compatible binder tested (established by its ability to form smooth films and aid in drug loading). When the SYN-004–HPC mixture was used to spray the active enzyme onto sugar cores, the spray application of about 220% by weight was required to effectively accomplish the desired SYN-004 load on the pellets. We hypothesized that the chemical contacts between the amino acid side chains on the outer surface of the SYN-004 enzyme and the hydroxypropyl moieties of the HPC binder enable the smooth surface as moisture evaporated from the liquid spray throughout the coating process. The process using HPC protected the biological activity throughout the manufacture of coated sucrose cores (11).
To provide gastric protection to the SYN-004 layered pellets, a formulation containing Eudragit L 30 D-55 copolymer and supplementary excipients glycerol monostearate, triethyl citrate, and polysorbate-80 was mixed to produce pellets with a smooth nontacky surface, free from cracks. The polymer application was about 30% of dry Eudragit L 30 D-55 copolymer sprayed on top of SYN-004 drug-layered pellets. That process yielded a coat thickness of 40–50 μm, which is considered sufficient to provide functional gastric protection. Dissolution profiles confirmed that the pellet formulation was protected completely during the two-hour acid stage because no activity was released into the medium, and about 50% of the SYN-004 activity was released within one hour following the change to pH 6.8 buffer, and >80% was released within four hours (11).
Ultimately, we expect a constant supply of active SYN-004 drug throughout the intestines with staggered release of pellets into the duodenum, the release profile, stability in intestinal chyme, and administration of SYN-004 capsules four times per day (11, 25). The SYN-004 drug loading in the pellets produced was about 16% w/w. This amount of active enzyme should provide a therapeutic concentration of SYN-004 drug from an oral dosage form designed to degrade β-lactam antibiotics excreted into the intestine.
SYN-010 Product Development
Lovastatin was approved in 1987 for lowering cholesterol, and it has a well understood safety profile. So it made a compelling candidate for reformulation and repurposing to treat IBS-C. Although the HA form is the cholesterol-lowering metabolite, Marsh et al. showed the lactone form inhibits methane production by methanogenic archaea in the intestines (25). The goal of reformulating lovastatin lactone was to reduce the cholesterol-lowering effect of lovastatin by limiting systemic absorption of HA while enabling its methane-lowering effect by delivering lovastatin lactone to methanogens in the intestines.
Patients with IBS-C are healthy enough to ingest solid oral dosage forms (26), so developing a tablet or capsule was a primary focus. Because M. smithii reside in the small and large intestines (27), and the undesirable conversion/absorption of lovastatin occurs primarily in the stomach and small intestines, we decided to release only a small amount of API in the small intestine and a larger quantity in the large intestine. To allow release of lovastatin in different compartments of the GI, we developed two tablet types to deliver together inside one capsule. The characteristics of crystalline lovastatin API and the required small therapeutic doses indicated that a dual-pulse formulation of lovastatin for IBS-C would be achievable in a reasonably sized capsule.
We used fine grades of direct-compression (DC) excipients in the blend to reduce the risk of size separation and settling before compression because lovastatin has a small particle size. A series of API blend and tableting trials determined which excipients worked best in terms of dry blend flow and compressibility. We batched recipes of excipients comprising sets of binders, disintegrants, dispersion agents, bulking agents, and lubricants with the lovastatin API and tableted them. Before compression of each experimental blend, we performed a Carr’s compressibility index test. One formulation tableted well and was used as the base formulation for later experiments.
We prepared formulations and tested them in ambient humidity at 50 °C, as well as 75% relative humidity at 5 °C. At ambient humidity and elevated temperatures, significant amounts of lovastatin were converted to the HA form. Considering the observation that lovastatin is susceptible to conversion to the HA metabolite in the presence of water, we investigated calcium carbonate DC (a moisture scavenger) as a possible solution. The moisture scavenger unexpectedly resulted in the conversion of lovastatin to the HA form in uncoated tablets. An alternative approach to control moisture, the use of a desiccant in a sealed container, prevented the degradation. We decided that the container–closure system should contain a desiccant pack to maintain SYN-010 HA impurity below 0.2% (9).
Loss during friability testing of the uncoated tablets was <1%, indicating that the tablets could withstand the erosion of the pan-coating process in commercial-scale equipment, packaging, and shipping. The disintegration results varied from seven minutes to >13 minutes. The target disintegration time was less than five minutes. We decided not to decrease the hardness to shorten the disintegration time because the tablet hardness was in a desirable range. Adjusting concentration of binder and disintegrant decreased the disintegration time to a desirable value. A tablet-hardness range was tested between 4 kp (kilopond) and 8 kp. In terms of friability and dissolution, all tablets were acceptable, so we set 6 kp as the hardness target.
We performed a binary excipient compatibility study that involved mixing individual excipients at a 1:1 ratio with lovastatin to evaluate the effect of each excipient on lovastatin stability. High moisture-content excipients correlated with increased degradation of the lovastatin lactone. From those results, we selected a lead tablet formulation and advanced into enteric coating studies.
The coating experiments included pan-coating studies with Eudragit L 30 D55 copolymer to release lovastatin in the duodenum (DR coating), and Eudragit FS 30D copolymer to release lovastatin in the ileocecal region (ICR coating) (14). Results from acid uptake experiments indicated that both enteric polymer selections were successful in protecting tablets from dissolving under acidic conditions. We obtained acceptable release kinetics using a coating level of 15% for both the DR and ICR tablets.
We assessed the lead coated tablet compositions using an in vitro dissolution method with a capsule of 42 mg containing one DR tablet and five ICR tablets. At pH 1.2 (simulating the stomach environment), no lovastatin was detected in the medium. At pH 5.9 (simulating the duodenum) about 7 mg of lovastatin derived from the DR tablet was measured in the dissolution medium, and the remaining 35 mg of lovastatin derived from the 5 ICR tablets was measured when the buffer pH was increased to pH 7.2 (9).
The dissolution profile predicted that upon ingestion, a 42-mg capsule would transit to the stomach, where the capsule would dissolve. The enteric-coated tablets would empty from the stomach intact through the pylorus and into the duodenum. The approximately pH 5.5 environment would cause the coating of the DR tablet to dissolve and release 7 mg (one tablet) of lovastatin lactone, inhibiting methanogenesis in the small intestine. The remaining ICR tablets would transit through the ileum until they reached a pH 7 environment, characteristically in the ileocecal region, upstream of the colon. At pH 7, we predict that these ICR tablets would dissolve, releasing the remaining 35 mg (five tablets) of lovastatin lactone to inhibit methanogenesis in the colon.
Strategies for Future Studies
The above two examples of formulation projects are useful because they have broad application and have been tested in vivo to verify that such formulations provide desirable properties. The SYN-004 product has been evaluated in multiple clinical trials (28, 29), which show that the drug was not absorbed systemically when orally delivered as a delayed-release pellet (24). Evidence shows that the drug product protects microbiomes from damage due to intravenous antibiotics, indicating that the β-lactamase is being protected from digestion in the stomach and is being released in the intestines to degrade the β-lactam antibiotic, as designed (30).
The SYN-010 drug also has been used in multiple clinical trials. A phase 2a clinical trial (NCT02495623) was completed with IBS-C patients. It showed oral administration of SYN-010 42 mg capsules decreased breath methane levels from day 1 to 12 weeks of treatment. Results also showed clinically important tendencies in the reduction of IBS-C symptoms (31). Fewer than half of patients in that study had detectable plasma trough levels of lovastatin and HA at days 7 and 28 (32) and were in keeping with the SYN-010 formulation design to deliver lovastatin lactone to the gut microbiome at two locations.
Numerous factors should be considered in the development of an oral dosage form for medicines targeting the gut microbiome. The challenges of excipient selection, API compatibility, enteric protection, and acceptable release profiles have multiple solutions that can be tested and optimized. The promising results of the human testing of some products described herein indicate that formulation strategies are effective and could be considered for formulating other therapies that target the intestinal microbiome. Those strategies are inevitable as studies of the intestinal microbiome continue to grow.
References
1 Liang D, et al. Involvement of Gut Microbiome in Human Health and Disease: Brief Overview, Knowledge Gaps and Research Opportunities. Gut Pathog. 10(3) 2018; doi:10.1186/s13099-018-0230-4.
2 Griffith DP, et al. Randomized, Double-Blind Trial of Lithostat (acetohydroxamic acid) in the Palliative Treatment of Infection-Induced Urinary Calculi. Eur. Urol. 20(3) 1991: 243–247.
3 Iebba V, et al. Eubiosis and Dysbiosis: The Two Sides of the Microbiota. New Microbiol. 39(1) 2016: 1–12.
4 Kaleko M, et al. Development of SYN-004: An Oral Beta-Lactamase Treatment to Protect the Gut Microbiome from Antibiotic-Mediated Damage and Prevent Clostridium difficile Infection. Anaerobe 41, 2016: 58–67. doi:10.1016/j.anaerobe.2016.05.015.
5 Pimentel M, Mathur R, Chang C. Gas and the Microbiome. Curr. Gastroenterol. Rep. 15(12) 2013: 356. doi:10.1007/s11894-013-0356-y.
6 Triantafyllou K, Chang C, Pimentel M. Methanogens, Methane, and Gastrointestinal Motility. J. Neurogastroenterol. Motil. 20(1) 2014: 31–40; doi:10.5056/jnm.2014.20.1.31.
7 McKay LF, Eastwood MA, Brydon WG. Methane Excretion in Man: A Study of Breath, Flatus, and Faeces. Gut 26(1) 1985: 69–74.
8 Muskal SM, et al. Lovastatin Lactone May Improve Irritable Bowel Syndrome with Constipation (IBS-C) By Inhibiting Enzymes in the Archaeal Methanogenesis Pathway. F1000Research 5, 2016: 606; doi:10.12688/f1000research.8406.3.
9 Hubert S, et al. Development of a Modified-Release Formulation of Lovastatin Targeted to Intestinal Methanogens Implicated in Irritable Bowel Syndrome with Constipation. J. Pharm. Sci. 107(2) 2018: 662–671; doi:10.1016/j.xphs.2017.09.028.
10 Mullish BH, Williams HR. Clostridium difficile Infection and Antibiotic-Associated Diarrhoea. Clin. Med. Lond. Engl. 18(3) 2018: 237–241; doi:10.7861/clinmedicine.18-3-237.
11 Bristol A, et al. Formulation Development of SYN-004 (Ribaxamase) Oral Solid Dosage Form: A β-Lactamase to Prevent Intravenous Antibiotic-Associated Dysbiosis of the Colon. Int. J. Pharm. 534(1–2) 2017: 25–34; doi:10.1016/j.ijpharm.2017.10.001.
12 Fallingborg J. Intraluminal pH of the Human Gastrointestinal Tract. Dan. Med. Bull. 46(3) 1999: 183–196.
13 Pinto JF. Site-Specific Drug Delivery Systems Within the Gastrointestinal Tract: From the Mouth to the Colon. Int. J. Pharm. 395(1–2) 2010: 44–52; doi:10.1016/j.ijpharm.2010.05.003.
14 Patra CN, et al. Pharmaceutical Significance of Eudragit: A Review. Future J. Pharm. Sci. 3(1) 2017: 33–45; doi:10.1016/j.fjps.2017.02.001.
15 Patra CN, et al. Osmotic Drug Delivery Systems: Basics and Design Approaches. Recent Pat. Drug Deliv. Formul. 7, 2013: 150–161.
16 Duttagupta DS, Jadhav VM, Kadam VJ. Chitosan: A Propitious Biopolymer for Drug Delivery. Curr. Drug Deliv. 12(4) 2015: 369–381; doi: 10.2174/1567201812666150310151657.
17 Desai PM, Liew CV, Heng PWS. Review of Disintegrants and the Disintegration Phenomena. J. Pharm. Sci. 105(9) 2016: 2545–2555; doi:10.1016/j.xphs.2015.12.019.
18 Nimkulrat S, et al. Influence of Selected Surfactants on the Tackiness of Acrylic Polymer Films. Int. J. Pharm. 287(1–2) 2004: 27–37; doi:10.1016/j.ijpharm.2004. 08.022.
19 Perrault M, Bertrand F, Chaouki J. An Experimental Investigation of the Effect of the Amount of Lubricant on Tablet Properties. Drug Dev. Ind. Pharm. 37(2) 2011: 234–242; doi:10.3109/03639045.2010.505013.
20 Lynch T, Price T. The Effect of Cytochrome P450 Metabolism on Drug Response, Interactions, and Adverse Effects. Am. Fam. Physician 76(3) 2007: 391–396.
21 Bezirtzoglou EEV. Intestinal Cytochromes P450 Regulating the Intestinal Microbiota and Its Probiotic Profile. Microb. Ecol. Health Dis. 23, 2012; doi:10.3402/mehd.v23i0.18370.
22 Ding X, Kaminsky LS. HumanExtrahepatic Cytochromes P450: Function in Xenobiotic Metabolism and Tissue-Selective Chemical Toxicity in the Respiratory and Gastrointestinal Tracts. Ann. Rev. Pharm and Toxicol. 43, 2003: 149–173; doi.org/10.1146/annurev.pharmtox. 43.100901.140251.
23 Jourova L, Anzenbacher P, Anzenbacherova E. Human Gut Microbiota Plays a Role in the Metabolism of Drugs. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 160(3) 2016: 317–326; doi:10.5507/bp.2016.039.
24 Kokai-Kun, et al. Nonclinical Safety Assessment of SYN-004: An Oral β-lactamase for the Protection of the Gut Microbiome from Disruption By Biliary-Excreted, Intravenously Administered Antibiotics. Int. J. Toxicol. 61(3) 2016: 309–316; doi:10.1177/ 1091581815623236.
25 Marsh E, et al. Lovastatin Lactone Inhibits Methane Production in Human Stool Homogenates. Poster at the American College of Gastroenterology Annual Meeting, 2016.
26 Herman J, et al. Gender Distribution in Irritable Bowel Syndrome is Proportional to the Severity of Constipation Relative to Diarrhea. Gend. Med. 7(3) 2010: 240–246; doi:10.1016/j.genm.2010.06.007.
27 Sachdev AH, Pimentel M. Gastrointestinal Bacterial Overgrowth: Pathogenesis and Clinical Significance. Ther. Adv. Chronic Dis. 4(5) 2013: 223–231; doi:10.1177/2040622313496126.
28 Kokai-Kun JF, et al. The Oral β-Lactamase SYN-004 (Ribaxamase) Degrades Ceftriaxone Excreted into the Intestine in Phase 2a Clinical Studies. Antimicrob. Agents Chemother. 61(3) 2017; doi:10.1128/AAC.02197-16.
29 Roberts T, et al. Tolerability and Pharmacokinetics of SYN-004, an Orally Administered β-Lactamase for the Prevention of Clostridium difficile–Associated Disease and Antibiotic-Associated Diarrhea, in Two Phase 1 Studies. Clin. Drug Investig. 36(9) 2016: 725–734; doi:10.1007/s40261-016-0420-0.
30 Pitout JDD. IPSAT P1A: A Class A Beta-Lactamase Therapy for the Prevention of Penicillin-Induced disruption to the intestinal microflora. Curr. Opin. Investig. Drugs Lond. Engl. 10(8) 2009: 838–844.
31 Gottlieb K, et al. Su1210 SYN-010, a Proprietary Modified-Release Formulation of Lovastatin Lactone, Lowered Breath Methane, and Improved Stool Frequency in Patients with IBS-C: Results of a Multi-Center Randomized Double-Blind Placebo-Controlled Phase 2a Trial. Gastroenterol. 150, 2016: S496–S497; doi:10.1016/S0016-5085(16)31709-7.
32 Wacher V, et al. SYN-010 Modified-Release Lovastatin Does Not Significantly Alter Lipid Parameters at Doses That Reduce Methane and Alleviate Symptoms in Patients Suffering Irritable Bowel Syndrome with Constipation (IBS-C). Abstract Am. J. Gastroenterol. 111, Supp. 1, 2016: S256. c
Corresponding author Dr. Andrew Bristol is vice president of development at Synthetic Biologics, Inc. Rockville, MD; 1-301-417-4364; [email protected]. Steven Hubert is senior director of manufacturing, and Raymond D. Stapleton, Jr. is senior vice president of technical operations.
You May Also Like





