Supplementing Gamma Sterilization with X-Ray and E-Beam Technologies: An International Industry and Academia CollaborationSupplementing Gamma Sterilization with X-Ray and E-Beam Technologies: An International Industry and Academia Collaboration
Ionizing-technology–based industries are growing rapidly around the world. The expansion is driven mostly by the technology’s myriad applications, including polymer crosslinking, medical device sterilization, food pasteurization, and phytosanitary treatment. Ionization also is used in the manufacture of some healthcare products such as medical devices and biopharmaceuticals. Industrial sterilization methods render single-use products and manufacturing components safe and ready for their intended use. ISO11137-1 describes the validation and routine control of a sterilization process for medical devices and mentions the three types of radiation considered under this scope: gamma, X-ray, and electron beam (e-beam) (1).

Figure 1: Examples of X-ray and electron-beam installations; scan horns are present in both installations. Sources: Steri-Tek (left) and Texas A&M University (right).
The Changing Sterilization Market Landscape
The global market for medical plastics will grow at a predicted compound annual growth rate (CAGR) of ~11% over the 2020–2025 period (2). As market demand and innovation increase for such materials, the demand for sterilization capacity and access to validated and approved sterilization methods will expand. A December 2016 report states that “the global sterilization market is expected to reach US $6.93 billion by 2021 from $4.69 billion in 2016, at a CAGR of 9%” (3). That market encompasses all sterilization methods, including steam, and takes into account the impacts of the current coronavirus pandemic. The International Irradiation Association has determined that the single-use medical device sterilization industry is about 40.5% gamma irradiation, 4.5% electron-beam (e-beam) radiation, 50% ethylene oxide (EO), and 5% the use of other modalities such as steam and X-ray (2). The association estimates that the global market for sterilization of single-use medical devices alone is already worth $6 billion.
Although the terminal sterilization of medical devices is about 3% of the total cost of the medical-device supply chain, without that step, the medical supply chain would come to a halt (4). The need for traditional sterilization methods (e.g., EO gas and gamma irradiation from radioactive cobalt-60) is ongoing. Simultaneously, the industry also has increased applications of e-beam and X-ray technologies.
The rising cost of cobalt-60 coupled with the healthcare industry’s and federal agencies’ recent reevaluation of EO as a sterilization modality have placed significant pressures on available sterilization capacity in the United States and Europe. Fortunately, alternative technologies based on e-beam and X-ray have matured significantly over the past decade in terms of their performance, capabilities, and robustness. Panoramic, industrial-sized equipment for both e-beam and X-ray technologies now is commercially available (e.g., from IBA Industrial and Mevex Corporation) and capable of delivering high energy (10 MeV) and high power (≥500 kW) to handle pallet-level sterilization. It is not surprising that the largest commercial sterilization providers are harnessing that availability and increasing their commercial sterilization capacity by using these machine sources in Europe, the United States, and elsewhere.
Minimizing Risks
Sterilization technology based on ionizing radiation still has some potential drawbacks, particularly in terms of whether the polymers can withstand the doses. Like any other sterilization technology, ionizing-technology–based methods are not compatible with all materials and thus can cause material and functional changes in devices. The effects of ionizing technology strongly depend on the dose, the modality (photons or electrons), and the specific material being sterilized. The geometry of the part and properties of the radiation environment also can play a role in the response of a material to the absorption of energy from high-energy electrons, X-rays, and gamma rays (5). The ionizing radiation energy for the three types of radiation considered is used to define the absorbed dose (in kGy) (1) because it is the ionizing ability of the radiation — the ability to ionize or kick electrons off the shell of atoms through Compton scattering (6) — that initiates the killing effect on microorganisms (7). When assessing the compatibility of a healthcare product to be sterilized by ionizing technology, detailed biocompatibility, material characterization, and functionality tests must be performed. All risks should be mitigated to define the optimal e-beam and X-ray doses that will be compatible with each device or product. In this regard, the Bio-Process Systems Alliance (BPSA) has published a successful, collective industry approach to implement alternative modes of irradiation sterilization to ensure business continuity in the rapidly growing single-use industry (8).
In Europe and the United States, several programs have been initiated by companies and/or government agencies to accelerate implementation to machine-sourced sterilization methods. The National Nuclear Security Administration (NNSA) in the United States has invested substantial resources to accelerate this implementation of machine sources, including the Team Nablo project led by the Pacific Northwest National Laboratory (PNNL). The Team Nablo project has brought together a consortium of stakeholders in the United States and Europe representing the healthcare products (medical devices and biopharmaceutical products) community, e-beam and X-ray equipment manufacturers, national laboratories, academic universities, and industrial R&D organizations. The team determines whether e-beam and X-ray irradiation modalities are comparable with cobalt-60–based irradiation for sterilization of medical devices. The project’s overall goal is to study the responses (material compatibility and functionality) of specific polymers used in the healthcare product industry to e-beam and X-ray irradiation doses. PNNL and Texas A&M University have partnered with medical device manufacturers such as Becton, Dickinson, and Company (BD), Stryker, and Sartorius to fill data gaps needed to assist the healthcare product industry in supplementing cobalt-60 irradiation with e-beam and/or X-ray technologies (9).
Characterization Strategy and Validation
To fill data gaps, the Team Nablo project has proposed a holistic research approach that covers several disciplines: nuclear technology, chemistry, physics, mechanics, materials, and mathematics. Such an approach already has been developed at a smaller scale by several members involved with the team. To achieve its goals, the team has integrated technological, academic, and industrial research at several levels (molecular, macromolecular, and materials levels) and has worked in conditions that are as close as possible to those found in industry.
Thus far, the team has evaluated the effects of gamma, e-beam, and X-ray irradiation doses on the characteristics of several polymers and medical device products. In one study, the team compared how sterilization-relevant doses (15, 35, 50, and 80 kGy) of gamma, e-beam, and X-ray irradiation affect low-density polyethylene (LDPE), polypropylene homopolymer (PPH), polyolefin elastomer (POE), and chlorobutyl rubber (CIIR) in terms of coloration and mechanical properties (10). Those specific polymers were selected because they represent the key components of Vacutainer Plus tubes (VT) and Vacutainer push-button blood-collection sets (PB) single-use blood collection devices from BD. More than five billion VT devices and 260 million PB devices are produced each year, and they are sterilized solely by gamma irradiation.
Out of 280 independent tensile, hardness, and discoloration tests, only 13% of results showed significant differences between existing gamma irradiation and either e-beam or X-ray technologies. In most of those different results, although the differences were statistically significant, only small changes in magnitude of the measured parameter were recorded. As such, these results do not necessarily indicate that the irradiated polymer does not meet performance specifications for its intended use. In this study, the most notable quantitative differences arose in the effects of e-beam and X-ray irradiation (relative to gamma) on the yellowness index (discoloration) for POE and PPH. Even those differences were not obvious to the human eye.
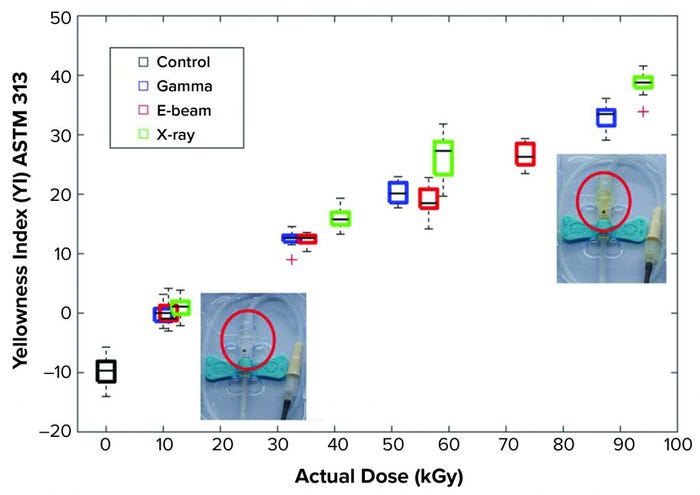
Figure 2: Yellowness index dose and modality dependency of a polypropylene homopolymer (PPH) material (red circled) (11).
Apart from the changes in yellowness index for POE and PPH, all statistical differences in the measured properties of gamma-irradiated and e-beam– and X-ray–irradiated polymers were recorded only in a subset of the four doses used for each material (typically only at a single dose). Thus, considering the entire range of doses for the three modalities, this study clearly showed that both e-beam and X-ray methods can substitute for gamma irradiation as effective sterilization options for the materials evaluated.
The team also assessed functional performance of the PB and VT medical devices after exposing them to sterilization-relevant doses (15, 35, 50, and 80 kGy) of gamma, e-beam, and X-ray irradiation (11). All devices passed the functional performance tests at all doses. Thus, both e-beam and X-ray irradiation are potentially viable alternatives to gamma irradiation for sterilization of medical devices and materials studied.
Sartorius and Aix-Marseille University developed a three-level approach for investigation of multilayer films and other materials (Figure 3). An analysis of the material properties at the product level focuses on those properties that are of the utmost importance for end users, including the integrity of the materials and the quality of the stored solutions. An analysis at the macromolecular level provides insights into the chemical changes leading to deterioration of material integrity. An analysis at the molecular level provides insight into all chemical processes leading to the observations made in the other two levels. The effects of gamma irradiation on the physical, mechanical, and chemical properties of multilayer films have been studied thoroughly using different techniques (12–17). Results of those studies will be used to assess the effects of X-ray and e-beam irradiations.
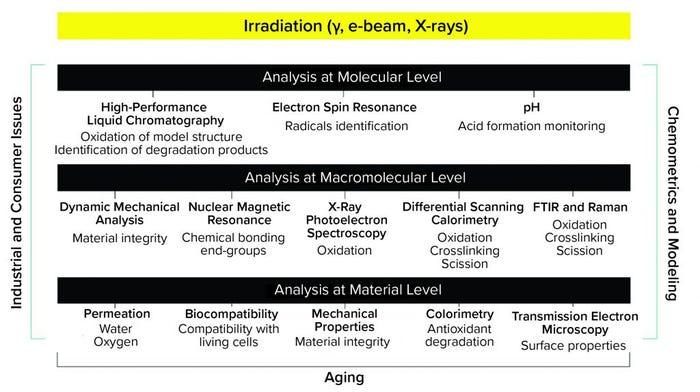
Figure 3: Multilevel testing approach to characterize materials after ionizing irradiations (gamma, X-ray, and electron beam).
When considering, for instance, Fourier-transform infrared spectroscopy coupled with attenuated total reflectance (FTIR-ATR) technique, out of the hundreds of spectra recorded, the intensity and location of the specific crystallinity bands (1474/1464 and 730/720 cm–1) do not vary with respect to dose. Therefore, the influence of gamma irradiation on the crystallinity of the EVA/EVOH/EVA (poly ethylene vinylacetate/poly ethylene vinyl alcohol) film is not large enough to be macroscopically detected by FTIR and is not large enough to affect the intended use of that material. By contrast, FTIR analysis combined with principal component analysis (PCA) of the EVA film surface shows a peak at 1714 cm–1 corresponding to the formation of carboxylic acid and a peak at 1739 cm–1 corresponding to the ester group degradation for increasing irradiation dose and overaging time (18). The effect of dose on the formation of carboxylic acid is significant only for doses >50 kGy. Those observations are taken as base observations to assess the influence of other ionizing radiations. Thus, for example, with a polyethylene / poly ethylene vinyl alcohol (PE/EVOH/PE) multilayer film as a model, we show that, whatever the type of irradiation, thermal properties are not significantly changed as shown by dynamical scanning calorimetry (DSC). Thus, physical and mechanical properties of that material also are expected to behave similarly (19). We also show with the PE/EVOH/PE multilayer-film model that the extractable profile is the same after gamma and X-ray (20) and that biocompatibility (ISO10993-5) remains likewise.
End users of sterilized materials are interested mainly in the properties of those materials and the impact of sterilization on manufactured products at the material level. Our team is investigating
• tribological properties (wear, friction, and lubrication)
• mechanical properties (stresses and strains)
• permeation to gases and liquids
• colorimetry (quality of materials)
• surface quality (roughness, oxidation)
• biocompatibility
• interactions with biopharmaceutical solutions (e.g., amino-acid oxidation processes and pH changes).
To propose solutions to end users, our team must investigate both at the macromolecular and molecular levels. That will help us to gain deeper insights into the elementary events that occur during material irradiation and during the aging of materials in the course of their use. At the macromolecular level, we are using dynamical mechanical analysis (DMA) and differential scanning calorimetry (DSC) to monitor changes in thermal properties of materials and changes in their integrity/stability. X-ray photoelectron spectroscopy (XPS) investigations provide information about chemical changes that occur on the surface of materials. Raman and FTIR-ATR provides further information about the chemical events that occur in polymer chains and the localization of those events in materials. Investigations at the molecular level will provide information about oxidation and crosslinking processes and degradation of antioxidants both immediately after irradiation and during aging under different conditions.
All changes observed at the macromolecular level originate from elementary events that occur at the molecular scale, and most of those events imply radical species. As the primary intermediates generated during irradiation processes, radical species are the primary source of phenomena at the macromolecular and materials levels. Thus, a comprehensive understanding of observations made at the macromolecular level requires further investigation at the molecular level. At that molecular level, we use different spectroscopic techniques to investigate the effects of irradiation (gamma, e-beam, and X-rays).
Electron-spin resonance (ESR) spectroscopy is highly useful because they can detect and quantify the presence of radicals. FTIR also provides data regarding radical processes that occur both during and after irradiation, including crosslinking, fragmentation, and oxidation processes. High-performance liquid chromatography (HPLC) methods detect and quantify molecular species after the degradation of antioxidants from materials. And pH analysis provides information about the acid release in solution from materials.
Such an investigation of materials may seem tedious, labor intensive, and time consuming. Fortunately, the different backgrounds of our team members provide robust expertise and capabilities in addressing different requests from the medical device and biopharmaceutical industries. Likewise, depending on the specific request, a more straightforward subset of the above experiments can be performed (8).
The approach proposed above generates huge amounts of data, and investigations often are focused on small changes at molecular and macromolecular levels that could induce large effects on a materialʼs properties. To help the team process those data, we use chemometrics methods, (e.g., principal component analysis, PCA) and design of experiments (DoE). Both strategies are intended for deep and thorough analyses and serve as predictive tools to help us select the best conditions of irradiation, storage, and aging that comply with sterilization requests of end users.
This international industry and academia collaboration has already started to generate data for supplementing gamma sterilization with X-ray and e-beam technologies.Preliminary results show no difference, for example, of the thermal properties and tensile strength after gamma and X-ray irradiations. Detailed results will be published in upcoming articles to get scientific data comparing gamma, X-ray, and e-beam technologies.
References
1 ISO 11137-1, Sterilization of health care products — Radiation — Part 1: Requirements for development, validation and routine control of a sterilization process for medical devices.
2 Medical Plastics: Global Markets. BCC Publishing: Wellesley, MA, 2020; https://www.bccresearch.com/market-research/plastics/medical-plastics-global-markets.html.
3 A Comparison of Gamma, E-beam, X-Ray and Ethylene Oxide Technologies for the Industrial Sterilization of Medical Devices and Healthcare Products. International Irradiation Association: Shropshire, UK, 2017.
4 Krocs T. Electron and X-Ray Sterilization of Medical Devices. GHPUDP, FDA Medical Device Advisory Meeting, 6 November 2019; https://www.fda.gov.
5 Fairand BP. Radiation Sterilization for Health Care Products: X-ray, Gamma, and Electron Beam. CRC Press: Boca Raton, FL, 2001.
6 Chapiro A. General Consideration of the Radiation Chemistry of Polymers. Nucl. Instr. Methods Phys. Res. 105, 1995: 5–7; https://doi.org/10.1016/0168-583X(95)00861-6.
7 Tallentire A, Miller A. Microbicidal Effectiveness of X-Rays Used for Sterilization Purposes. Rad. Phys. Chem. 107, 2015: 128–130; https://doi.org/10.1016/j.radphyschem.2014.09.012.
8 X-Ray Sterilization of Single-Use BioProcess Equipment, Part 1: Industry Need, Requirements, and Risk Evaluation. Bio-Process Systems Alliance, 2021; https://bpsalliance.org/wp-content/uploads/2021/X-Ray-White-Paper/FINAL-BPSA-X_Ray-Sterilization-of-SU_051321.pdf.
9 Hathcock J. X-Ray Sterilization of Single-Use Bioprocess Equipment: Materials Impact Assessment. PharmaEd Resources Extractables & Leachables Summit, June 2021.
10 Fifield LS, et al. Direct Comparison of Gamma, Electron Beam, and X-Ray Irradiation Doses on Characteristics of Low-Density Polyethylene, Polypropylene Homopolymer, Polyolefin Elastomer, and Chlorobutyl Rubber Medical Device Polymers. Radiation Phys. Chem. 186, 2021: 109505; https://doi.org/10.1016/j.radphyschem.2021.109505.
11 Fifield LS, et al. Direct Comparison of Gamma, Electron Beam, and X-Ray Irradiation Effects on Single-Use Blood Collection Devices with Plastic Components. Radiation Phys. Chem. 180, 2021: 109282; https://doi.org/10.1016/j.radphyschem.2020.109282.
12 Audran G, et al. Degradation of γ-Irradiated Polyethylene–Ethylene Vinyl Alcoholpolyethylene Multilayer Films: An ESR Study. Polymer Degradation and Stability 122, 2015: 169–179; https://doi.org/10.1016/j.polymdegradstab.2015.10.021.
13 Gaston F, et al. Impact of γ-Irradiation, Ageing and Their Interactions on Multilayer Films Followed By AComDim. Analyt. Chim. Acta 981, 2017: 11–23; https://doi.org/10.1016/j.aca.2017.05.021.
14 Gaston F, et al. One-Year Monitoring By FTIR of γ-Irradiated Multilayer Film PE/EVOH/PE. Radiation Phys. Chem. 125, 2016: 115–121; https://doi.org/10.1016/j.radphyschem.2016.03.010.
15 Girard-Perier N, et al. Study of the Mechanical Behavior of Gamma-Irradiated Single-Use Bag Seals. Food Packaging and Shelf Life 26, 2020: 100582; https://doi.org/10.1016/j.fpsl.2020.100582.
16 Samuel Dorey, et al. XPS Analysis of PE and EVA Samples Irradiated at Different γ-Doses. Appl. Surface Sci. 427, Part B 2018, 966–972; https://doi.org/10.1016/j.apsusc.2017.09.001.
17 Dorey S, et al. Reconciliation of pH, Conductivity, Total Organic Carbon with Carboxylic Acids Detected by Ion Chromatography in Solution After Contact with Multilayer Films After γ-Irradiation. Euro. J. Pharm. Sci. 117 (2018) 216–226; https://doi.org/10.1016/j.ejps.2018.02.023.
18 Gaston F, et al. Investigations at the Product, Macromolecular, and Molecular Level of the Physical and Chemical Properties of a Gamma-Irradiated Multilayer EVA/EVOH/EVA Film: Comprehensive Analysis and Mechanistic Insights. Polymers 13(16) 2021: 2671; https://doi.org/10.3390/polym13162671
19 Girard-Perier N, et al. Effects of X-Ray, Electron-Beam and Gamma Irradiation on PE/EVOH/PE Multilayer Film Properties. Chem. Com. 84, 2021; https://doi.org/10.1039/d1cc02871e.
20 Menzel R, et al. X-Ray Sterilization of Biopharmaceutical Manufacturing Equipment: Extractables Profile of a Film Material and Copolyester Tritan Compared to Gamma Irradiation. Biotechnol. Prog. 2021: e3214; https://doi.org/10.1002/btpr.3214.
Corresponding author Nathalie Dupuy ([email protected]) is professor of spectroscopy and chemometrics at Aix Marseille University, Avignon University, France. Corresponding author Sylvain R.A. Marque ([email protected]) is professor in radical spectroscopy and organic chemistry at Aix Marseille University. Leonard S. Fifield is senior research scientist at Pacific Northwest National Laboratory (PNNL), Richland, WA. Matt Pharr is an assistant professor in the department of mechanical engineering at Texas A&M University, College Station, Texas. David Staack is associate professor in the department of mechanical engineering at Texas A&M University. Suresh D. Pillai is director of the National Center for Electron Beam Research and Professor of Molecular Microbiology at Texas A&M University. Larry Nichols is chief executive officer at Steri-Tek. Corresponding author Mark K. Murphy ([email protected]) is senior research scientist, radiation measurements and irradiations, at PNNL. Corresponding author Samuel Dorey ([email protected]) is principal scientist, materials and irradiation, at Sartorius, France.
You May Also Like





