- Laboratory Equipment
- Sponsored Content
Using Slope Spectroscopy Methods: Risk Assessment and Cost SavingsUsing Slope Spectroscopy Methods: Risk Assessment and Cost Savings
Sponsored Content
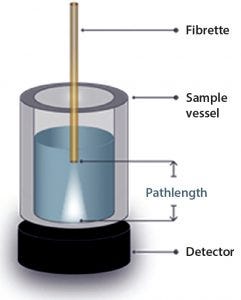
Figure 1: Sample configuration
The biopharmaceutical industry’s need for rapid, accurate concentration measurements of protein-containing products is critical. The protein-concentration assay measures ultraviolet absorption at 280 nm (A280) and usually is performed both as an in-process test and for product-release testing. The SoloVPE system can analyze samples across a wide range of target concentrations without the need for labor-intensive and error-prone dilutions. Slope Spectroscopy methods provide companies with a universal platform for determining protein concentration for all in-process, clinical, and commercial methods.
During in-process UV testing, turn-around time is as important as accuracy as analysts must wait for results before they can continue processing. Traditional methodologies relying on fixed-pathlength UV spectroscopy can require several hours of stagnant time because of the need for careful sample handling, preparation, and (in particular) dilutions needed for bringing samples into the spectrophotometer’s linear range. Errors created in performing those dilutions can affect calculated sample concentration significantly.
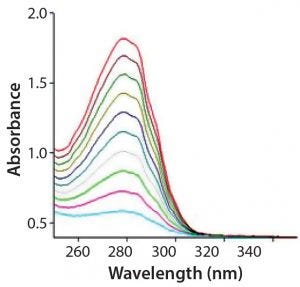
Figure 2: Variable pathlength spectra
Accelerating in-process development and manufacturing activities is crucial to remaining competitive in today’s pharmaceutical industry. This requires strong collaboration with vendors for analytical method development, including potential outsourcing partnerships. Our combined goal is to provide evidence of superior performance of variable-pathlength technology over the established methodology. Biogen’s assessment shows why companies should embrace Slope Spectroscopy technology as their primary platform for concentration determination.
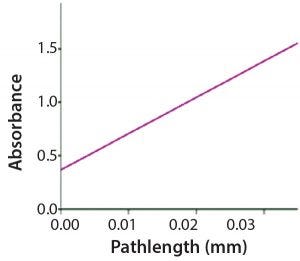
Figure 3: Slope regression
Apparatus and Equipment
The SoloVPE system and Slope Spectroscopy methods precisely vary the measurement pathlength to create a section (absorbance/pathlength) plot that complies with Beer’s law. The data slope is directly proportional to sample concentration. With micron pathlength resolution, this method allows for measurements of even the most highly concentrated samples without dilution and frequently without baseline correction. Minimum slope R2 values of 0.999 provide pass–fail criteria for samples measured (Figures 1–3).
Efficiency
Implementation and Training: With a general understanding of Beer’s law, SoloVPE operation requires no specialized technical experience or knowledge. Based upon its simplicity, the system can be used in any areas where a pH or conductivity meter might otherwise support real-time testing. With a brief introduction to the instrument, an analyst can start training within minutes. Operation generally should be identical no matter which department or laboratory uses the instrument. Unlike with alternative concentration-measurement methods, an analyst can learn to maintain the instrument and run standards and samples, all within an hour.
Reducing Sample/Buffer Preparation Time: Inclusion of variable-pathlength acceptance in the recently revised ASTM E169-16 standard allows for adjustment of pathlength in optimization of absorbance to avoid the dilution step. Slope Spectroscopy technology does not provide an absolute absorbance measurement; the variable pathlength technique monitors the change of absorbance from pathlength to pathlength. Most formulation buffers typically have a slope <0.01, requiring no baseline correction.
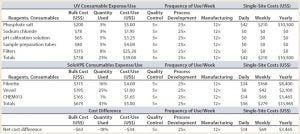
Table 1: Cost savings in materials — comparing SoloVPE analysis with classic UV-Vis spectroscopy
Standard Platform: Minimization of analyst intervention leads to better consistency between process steps, laboratories, and sites. System suitability and sample measurements in triplicate, can be tested within 15 minutes. Transfer between laboratories, sites, and companies is extremely efficient.

Table 2: Cost savings in time — costs based on additional analyst time committed to UV analysis
A Method for Efficiency
Significant efficiency gains are made possible by using the SoloVPE system (Tables 1 and 2). Based on those results, the following overall savings can be achieved (in US dollars):
Daily $1,234
Weekly $6,168
Yearly $318,465.
Slope Spectroscopy analysis requires no sample preparation or dilution, saving substantial time without changing any other aspect of the assay. A single SoloVPE system provided >50% overall cost savings in instrument time for samples analyzed using Slope Spectroscopy methods. Additionally, this assessment captured no potential manufacturing or laboratory time for investigating facility issues. The study does not compare additional time spent waiting for results generated by traditional UV analysis.
Processes that involve dilution requirements represent the highest single source of error in traditional UV–Vis analysis. Eliminating laboratory inconsistencies such as dilution error, external calculations, limited linear range, sample preparation, and handling makes the SoloVPE system magnitudes superior to traditional fixed pathlength UV technology.
Several years of acquired data, validated methods, system robustness documentation, and regulatory acceptance have paved the way for a more accurate methodology such as Slope Spectroscopy analysis to be used as a platform method to replace UV technology for all groups in pharmaceutical organizations.
Further Reading
1 DOC0057(EN): SoloVPE System Specifications. C Technologies Inc., Bridgewater, NJ, 2011; https://ctechnologiesinc.com/sites/default/files/2018-05/DOC0057_SoloVPE_Specification_Sheet.pdf.
2 ASTM E169-16: Standard Practices for General Techniques of Ultraviolet–Visible Quantitative Analysis. ASTM International: West Conshohocken, PA, April 2016; https://ctechnologiesinc.com/sites/default/files/2017-12/ASTM-E0169-16.pdf.
Jason Matthews is manager of analytical technology quality control chemistry at Biogen ([email protected]). Corresponding author Joe Ferraiolo is applications manager at C Technologies Inc. ([email protected]). Slope Spectroscopy and SoloVPE are registered trademarks of C Technologies Inc.
You May Also Like





