Viral-Vectored Gene Therapies: Harnessing Their Potential Through Scalable, Reproducible Manufacturing ProcessesViral-Vectored Gene Therapies: Harnessing Their Potential Through Scalable, Reproducible Manufacturing Processes
Adenoassociated virus (AAV) often serves as a vector for gene therapies. (www.istockphoto.com)
We might not associate the jazz queen Ella Fitzgerald with 21st-century gene-based therapies, but the First Lady of Song was on to something back in 1939 when she sang “’T’Ain’t What You Do (It’s the Way That You Do It).” Although demonstrating the safety and efficacy of gene-based therapies in rigorous clinical trials is essential for gaining product approval from regulators, doing the bare minimum is insufficient. The way that such products are produced also matters. Manufacturing processes and protocols that assure safety, purity, and consistency of gene-based therapies are critical to the approval process, whereas a cost-effective manufacturing process is essential for commercial success.
US Food and Drug Administration (FDA) approvals of Luxturna (voretigene neparvovec-rzyl) for treatment of RPE65 mutation-associated retinal dystrophy and Zolgensma (onasemnogene abeparvovec-xioi) for SMN1-related spinal muscular atrophy have validated adenoassociated virus (AAV)-based gene therapies as transformative approaches to treating diseases that have significant unmet needs. A growing pipeline of late-stage gene-therapy clinical trials for diverse indications — including choroideremia, hemophilia B, mucopolysaccharidosis type IIIA, X-linked retinitis pigmentosa (XLRP) and achromatopsia — is expected to yield additional product approvals over the next several years.
Manufacturing Challenges
Despite validation of AAV-vectored gene therapy as an effective treatment modality across multiple disease indications, manufacturing continues to pose a challenge to expanding the portfolio of approved AAV-based products. That is due to the complexity of producing recombinant AAV vectors, which requires simultaneously introducing DNA sequences encoding the AAV replication (rep) and capsid (cap) genes, a therapeutic gene, and helper-function genes that are essential for AAV replication into a single cell. Cells that receive the rep, cap, and helper-function genes but lack the therapeutic gene could yield empty vectors that lack therapeutic potential (Figure 1A).
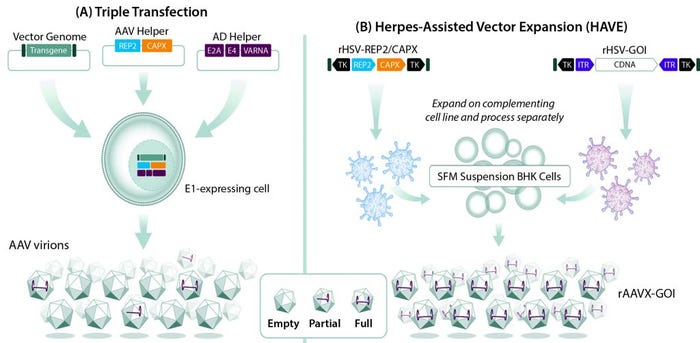
Figure 1: Comparing (A) triple transfection and (B) herpes-assisted vector expansion (HAVE) production of recombinant adenoassociated virus (AAV) vectors; during triple transfection, a transgene of interest, genes encoding AAV replication (rep) and capsid (cap) proteins, and genes encoding adenovirus helper functions (E2A, E4, and VARNA) are isolated on separate plasmids and transfected into an adenovirus E1-expressing cell line. Not all cells receive all three plasmids, resulting in a mixed population of empty, partial, and full AAV vectors. In a HAVE method, recombinant herpes simplex virus (HSV) vectors carrying rep and cap sequences and a gene of interest (GoI) are introduced into baby hamster kidney (BHK) cells grown in suspension. That results in populations with much higher proportions of full AAV vectors.
Several AAV manufacturing processes use adherent cells, which must be grown in contact with a bottle or tissue-culture plate surface. An inherent limitation to such processes is that they can be scaled out (through increasing surface area) but not scaled up (by increasing volume). That means production can be augmented only by increasing the number of culture vessels such as stacked trays or roller bottles, which significantly increases physical space requirements and drives up manufacturing costs (Figure 2A). Furthermore, managing and processing many vessels requires additional personnel and increases risks for contamination and processing errors.
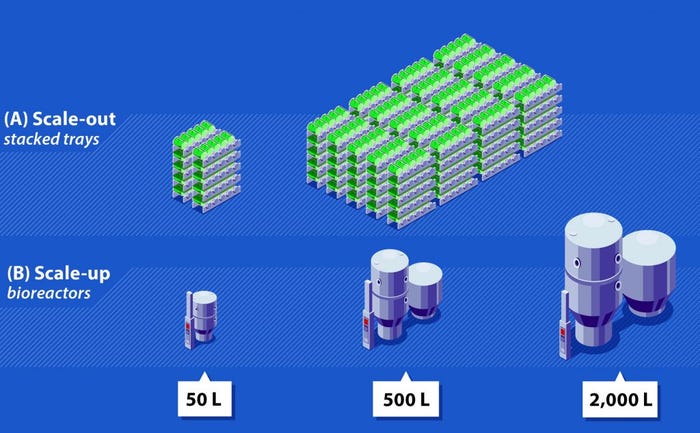
Figure 2: Comparison of scale-out and scale-up; (A) Scale-out with stacked-tray technology and roller-bottle–based manufacturing methods requires a considerable number of vessels, increasing both manipulation and footprint requirements; (B) scale-up with bioreactor-based manufacturing methods can be achieved by increasing reactor size, which will reduce footprint, manipulation, and labor requirements significantly.
An Urgent Need
Even with such limitations, many laboratory and industry AAV manufacturing processes continue to rely on transient transfection of adherent cells. Although adherent processes might support preclinical and early clinical development, they typically are not robust enough for late-stage clinical or commercial manufacturing needs. That is because they tend to produce a low ratio of active vector per volume of tissue culture. High volumetric productivity is essential for developing a cost-effective AAV manufacturing process that can meet late-stage and commercial needs. Such processes also could allow for reduced reagent volumes, which in turn could lower reagent costs and facility size. Scalability is important to addressing rare diseases and low-dose indications from a cost perspective while attending to more common indications from a feasibility perspective.
Limitations with adherent-cell manufacturing processes demand alternative approaches. Baculovirus- and adenovirus-based methods that rely on cells grown in suspension enable scalable manufacturing processes that use bioreactors commonly operated for production of recombinant-protein and antibody therapies under good manufacturing practice (GMP) conditions. Yet those approaches have their own limitations. Production of mammalian viral vectors in insect cells using the baculovirus expression vector system (BEVS) can create challenges such as AAV vectors with reduced infectivity (1). Producer cell lines that are transfected stably can produce AAV through adenovirus infection, supporting efficient and large-scale AAV manufacturing. However, each new gene therapeutic requires a unique producer cell line, reducing efficiency of the approach overall (2).
Herpes simplex virus (HSV) (www.istockphoto.com)
Vector-Expansion Solutions
Herpes-assisted vector expansion (HAVE) provides a robust, scalable, and highly productive method for manufacturing AAV vectors. Herpes simplex virus (HSV) vector production of AAVs overcomes the limitations of baculovirus-based systems by using mammalian cells, and HSV vectors eliminate the need to develop multiple producer cell lines otherwise required with adenovirus-based systems.
In a HAVE approach, baby hamster kidney (BHK) cells in suspension culture are coinfected with two recombinant HSV vectors: one carrying the AAV rep and cap genes and the other carrying the therapeutic gene of interest flanked by AAV inverted terminal repeat (ITR) sequences. This approach can tailor selection of the cap gene sequence for optimum cell targeting and transduction. HAVE systems work with multiple AAV serotypes in a plug-and-play fashion, including a proprietary variant of the AAV2 capsid in which three tyrosine residues have been changed to phenylalanine to enhance retinal-cell transduction. A HAVE approach can result in a 50-fold increase in productivity compared with transient transfection of the same recombinant sequences (Figure 1B). With aggressive scaling, HAVE also is expected to decrease cost of goods (CoG) 90-fold. Such a decrease would result from several aspects of the HAVE process, including
the ability to scale up production with bioreactors rather than by scaling out with additional adherent vessels (Figure 2B)
increased upstream productivity and improved downstream recovery, yielding a low percentage of empty vectors.
The ability of a single HAVE-produced lot to meet demand for an early stage clinical trial further confirms the merit of this manufacturing approach.
HAVE processes offer several clinical and regulatory advantages over other AAV-production methods. First, they have demonstrated chemistry, manufacturing, and controls (CMC) success in early clinical development. System adaptations have provided material for six successful investigational new drug (IND) applications without any clinical holds. HAVE helper-construct vectors show strong stability. Researchers have been able to develop and transfer product-specific assays to contract research organization (CRO) partners. Entire HAVE processes have been transferred successfully to industry partners and contract manufacturing organizations (CMOs).
Second, BHK cells and Vero-based cells used to produce recombinant HSV vectors have been validated through their uses in manufacturing therapeutic proteins and vaccines. The FDA and other national regulatory agencies also have reviewed HAVE processes.
Not Only How, But When
Although the Queen of Jazz was right to emphasize “the way that you do it,” the “when” of AAV manufacturing also is critical to clinical, regulatory, and commercial success. Many promising gene-therapy programs originate in academic laboratories that use small-scale, low-productivity AAV-vector manufacturing methods. Such methods might be appropriate for academic infrastructure and financial resources and generate material for preclinical animal studies, but they often lead to challenges down the road.
Biopharmaceutical companies that in-license such programs typically are pressured to move quickly into early phase human clinical trials. So they are reluctant to invest in high-productivity manufacturing processes and facilities before generating positive phase-2 clinical data suggestive of longer-term clinical success and commercial viability. That reluctance appears to make sense from a resource-use perspective, but in the long term it could create a significant gap between manufacturing capabilities and clinical and regulatory demands. Changing a manufacturing process later in clinical development, even when a change is beneficial for increasing productivity, can lead to significant delays in initiating or completing pivotal trials unless the change is implemented well in advance of material needs. Developing robust characterization assays is essential for navigating a manufacturing process change successfully because their results must demonstrate that the end products are comparable even if the processes used to produce them are different.
Applied Genetic Technologies Corporation (AGTC) invested in its HAVE platform early in the development of its clinical pipeline, addressing manufacturing productivity and quality as a foundational starting requirement rather than a future need. This strategy has eliminated vector production and testing as barriers to entering clinical development across the company’s development pipeline. It also has enabled development of manufacturing capabilities that go beyond the needs of inherited retinal diseases to support a broad set of indications with diverse vector requirements.
As the biopharmaceutical industry works to make AAV-based gene therapies a readily available approach to addressing unmet clinical need in a growing array of indications, manufacturing becomes increasingly critical. Scalable, high-productivity approaches like the HAVE method implemented early in development are “the way to do it” if you want long-term success.
References
1 Smith DC. AAV Vector Manufacturing: Challenges and Opportunities in the Manufacturing of AAV Vectors Used in the Delivery of Gene Therapy Treatments. Drug Develop. Deliv. 17(2) 2017: 18–26; https://drug-dev.com/aav-vector-manufacturing-challenges-opportunities-in-the-manufacturing-of-aav-vectors-used-in-the-delivery-of-gene-therapy-treatments.
2 Naso MF, et al. Adenoassociated Virus as a Vector for Gene Therapy. BioDrugs 31(4) 2017: 317–334; https://doi.org/10.1007/s40259-017-0234-5. c
Sue Washer is president and chief executive officer, and David R. Knop, PhD, is executive director of process development at Applied Genetic Technologies Corporation (AGTC), 14193 NW 119th Terrace, Suite 10, Alachua, FL 32615; 1-386-462-2204.
You May Also Like






