- Manufacturing
- Sponsored Content
Challenges in the Development of Biologic Drug ProductsChallenges in the Development of Biologic Drug Products
August 11, 2016
Sponsored by Lonza Biologics
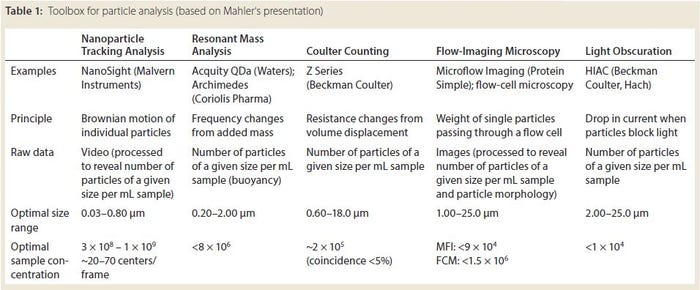
Hanns-Christian Mahler (head of drug product services, Lonza), BPI Theater @ BIO, June 8, 2016 11:20–11:40 am
 A drug product transfers a biomolecule (drug substance) into a form that will be usable by patients. It contains the active ingredient with excipients and can include packaging or a device for administration. In many cases, patients must be able to administer drugs to themselves. “Think about how the drug will be administered before designing your formulation,” Mahler suggested.
A drug product transfers a biomolecule (drug substance) into a form that will be usable by patients. It contains the active ingredient with excipients and can include packaging or a device for administration. In many cases, patients must be able to administer drugs to themselves. “Think about how the drug will be administered before designing your formulation,” Mahler suggested.
Keeping a biomolecule intact is a challenge during both manufacturing and integration of drug and application device. Other challenges encountered during development might include protein stability, protein aggregation, and particle formation. Companies must find primary packaging that will not interact with their formulations.
High-concentration formulations are necessary in cases such as injections to the eye, a location where only small volumes can be used without increasing eye pressure. High concentrations are especially challenging to maintaining stability (the chance of aggregation or particle formation is increased), to manufacturing (higher viscosity is harder to filter), and to administration (higher viscosity products are harder to inject with small-diameter needle).
Regulatory requirements are evolving for drug products, Mahler said. “Be prepared for questions on formulation design, particulates, patient administration, and container– closure integrity.”
Drug products are an underappreciated area of biologic drug development. High-concentration products have their own challenges in manufacturing, stability, and administration. Particulates are a typical challenge and of increasing interest to regulatory agencies. Develop drug products with an integrated approach that considers formulation, manufacturing, primary packaging, and the administration device together.
You May Also Like






