- Manufacturing
- Sponsored Content
Delivering Unique Sterile Primary Container Closures with Advanced Aseptic ManufacturingDelivering Unique Sterile Primary Container Closures with Advanced Aseptic Manufacturing
August 11, 2016
Sponsored by Catalent
Bill Hartzel (director of strategic execution, Catalent Pharma Solutions) BPI Theater at INTERPHEX, April 27, 2016 11:00–11:20 am
How do biopharmaceutical companies leverage technology to provide unique primary container– closures to the market space? Catalent uses an advanced aseptic process of blow–fill–seal (BFS) technology. Adding automation makes for an efficient process that can reduce or eliminate human intervention needed in critical drug-product filling applications. Catalent’s system uses one automated machine in which a container is quickly formed, filled, and sealed within the confines of the same machine. The biggest advantage of this plastic-based system over glass vials is flexibility in the design of closures. Some unique example designs include oral closures for pediatric medicines, respiratory misters, and eye droppers.
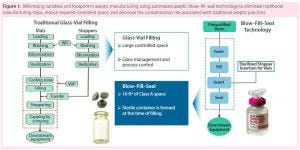
A BFS Process: Raw plastic resin is melted at a high temperature, then formed into a molded tube (the primary container). In the first stage, the bottom of that container is formed and cooled while the top remains pliable. In under two seconds, the container moves to stage two for filling. In stage three, a closure is formed through injection molding, when the container is sealed. These containers and closures are leak-proof and sterile, with good integrity.
Catalent compared its containers to those of other companies and found that the BFS method drastically reduces the amount of particulates present — even compared with glass. These containers also meet appropriate standards for extractables and leachables.
The company also polled nurses about their preferences as part of its market research. And nurses did see benefits to this system over using glass vials with elastomeric stoppers.
Hartzel concluded with an aphorism often credited to Leonardo da Vinci: “In simplicity is your ultimate form of sophistication.” He described Catalent’s automated filling system as a good example: a simple, efficient process that eliminates much variability and gives patients a simple way to take their medications.
Questions and Answers
What temperatures are drug products subjected to in this filling process? The resin is melted at 180 °C, but each new container cools quickly to 23–40 °C before product is filled.
How commonly used is this technology? Many companies overseas and in Mexico are already using BFS.
What are some design limitations (e.g., container size, number of products filled)? There are some limitations in design because you can’t have long fillling cycles. Because the containers are extrusion blown, the container size is limited to a liter maximum.
Listen to the full presentation at www.bioprocessintl.com/Interphex2016
You May Also Like





