Emerging Treatments for Spinal Cord DamageEmerging Treatments for Spinal Cord Damage
September 21, 2019
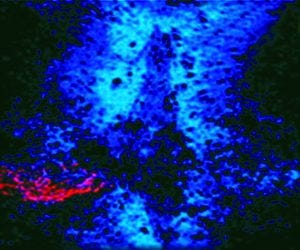
Photo 1: In this spinal cord lesion, red-dyed spinal cord nerves are trapped in a glial scar by blue-dyed CSPG proteins.
Injuries to the spinal cord can cause permanent paralysis and even lead to death, with little or no hope for patients to regain lost function after such trauma has occurred. News of my spinal cord research first came to prominence in the 1980s with a front-page story that chronicles a presentation at the annual Society for Neuroscience meeting (1). That reported the first time that crushed peripheral nerves had been regenerated back into a patient’s spinal cord.
Regeneration of spinal cord nerves after injury — a far more complex challenge — is coming closer to fruition as Canadian company NervGen Pharma works to develop my discoveries with an interim goal of conducting human clinical trials. At Case Western Reserve University’s medical school, my team and I are working to understand why nerves that are damaged through spinal cord injury (SCI) don’t regenerate and to identify noninvasive, easy-to-administer strategies for promoting robust functional recovery.
Can Damaged Nerves Regrow?
My research began with an investigation of whether it was possible for an adult nerve cell to regrow within the environment of a damaged adult spinal cord. Experts had thought for some time that it was impossible. Nerve cells have elaborate long extensions (axons) that grow out from their main cell bodies. The axons connect nerve cells through synapses which are severed within the spinal cord when a person’s bony spine is impelled into the cord through a violent break. Axons can be long, some stretching nearly the entire span of our bodies. One challenge for researchers working in the field of spinal injury had been to determine whether damaged axons have an intrinsic capacity (that is, a strong enough growth motor) for possible replacement.
My team and I found that such replacement indeed was possible, even long after traumatic damage had occurred. To demonstrate that, we conducted a simple experiment: First, we purified in cell culture fully adult nerve nells that were genetically prelabeled with a fluorescent protein so they could be visualized easily. That first step served not only to harvest the nerve cells, but also to cut all the axons away from their cell bodies, which nevertheless remained alive. Next, we collected the axon-free nerve-cell bodies and gently reimplanted them into the prelesioned spinal cord of an unlabeled adult host animal without damaging or scarring the implant site.
It was a surprise when we found that axons could grow robustly in the spinal cord — and reach out quickly over long distances. However, when those new axons reached the area of severe lesion damage and scarring, they stopped growing abruptly and began to deteriorate. We suspected that a chemical produced by scar tissue around the break itself was hostile enough to axons that it stops even exuberantly growing new ones from extending farther.
Understanding Scar Tissue
Inflammatory spinal cord damage after an injury can spread outward from a lesion’s epicenter into surviving tissue, causing further loss of function. Developing scar tissue encases the lesion core and plays an important role in protecting healthy nerve cells from further harm. However, once that important task of protection is complete, the same structure that offered such protection ultimately develops into a barrier to the regrowth of severed axons.
My team and I set out to identify the culprit and try to reduce or overcome the growth-inhibitory effect of such scars once they have mitigated the danger of an initial injury. Having shown already that axons can regrow if they can get past or through a scar, we understood that this could be the key to returning or improving function to injured areas.
We discovered a family of potentially inhibitory molecules produced in the scar tissue. When newly regenerating axons encountered them, the growing tips of those axons became so tightly stuck (like a fly on flypaper) that they could move forward no longer. These sticky molecules are known as chondroitin sulphate proteoglycans (CSPGs). Photo 1 illustrates this by showing a spinal cord lesion where the red-dyed spinal cord nerves are trapped in a glial scar by the blue dyed CSPG proteins (2).
Bridging the Scar Tissue to Repair Nerve Damage

Figure 1: Protein tyrosine phosphatase sigma (PTPσ) receptor at the end of a neuron binds and locks to the chondroitin sulphate proteoglycans (CSPG) protein matrix of a glial scar in an injured spinal cord.
After determining what stops the growth of axons near scar tissue, we hoped to find a way to selectively eliminate CSPGs. We investigated the enzyme chondroitinase, which consumes CSPGs when injected into a spinal cord, thus stopping them from having such detrimental effects. Using the enzyme, we regrew axons and improved function in animals that had problems with their limbs, bladders, and diaphragms. Such problems are common and potentially fatal conditions in humans following paralysis, so they were especially promising targets for improvement.
Use of enzymes to break down CSPGs has been investigated widely, with researchers attempting to optimize factors such as the timing of administration and appropriate amount of targeted physical therapy. Benefits have been shown in a number of animal models, including non-human primates, and at long chronic timepoints following injuries. However, the enzyme is of bacterial origin, must be injected directly into an injured spinal cord, and acts only over a very short distance. Supplementary treatments also could be required to ensure optimal spread of the enzyme and help regrown axons make the right connections. Studies are ongoing, with a number of research teams around the world seeking to overcome these challenges.
To eliminate the need for direct injection into a damaged spinal cord, my colleagues and I tested the effects of a novel systemic approach on axon regrowth. We identified a receptor molecule on the axons called protein tyrosine phosphatase sigma (PTPσ) that acts to help CSPGs be overly adhesive (3). Figure 1 shows an injured spinal cord and illustrates how the PTPσ receptor at the end of a neuron binds and locks to the CSPG protein matrix of a glial scar.
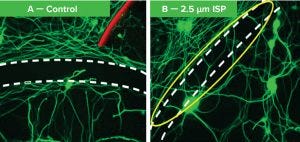
Figure 2: At left, the area between the dotted lines has a high concentration of chondroitin sulphate proteoglycans (CSPGs), so neurons are stopped from growing across. At right, many neurons have crossed the CSPG area after addition of intracellular sigma peptide (ISP). They no longer recognize the scar as a barrier and grow through it.
This newly discovered receptor provides a way for axons to detect CSPGs and signals the axons to stop and become entrapped. We developed an intracellular sigma peptide (ISP) molecule that negates that signal, allowing the regenerating axons to ignore and bypass CSPGs. When ISP was administered noninvasively by injection under the skin, it interfered with the CSPG/receptor signaling within the spinal cord, allowing for robust axon regrowth that greatly improved bladder function and locomotion in animal models of spinal cord injury (4–8). Figure 2 illustrates such results.
About the Product
In development as a biopharmaceutical product at NervGen Pharma, ISP is known as NVG-291. It is a linear peptide manufactured synthetically using established procedures by an approved contract manufacturing organization (CMO), and NervGen plans to have a secondary source available by the time the product goes commercial. This peptide is composed of common amino acids and has been produced in small quantities for research studies so far. Materials to be used in clinical trials will be manufactured by an approved CMO under current good manufacturing practice (CGMP) regulations. Several research batches of peptide-based PTPσ inhibitors have been manufactured. The plan is to administer NVG-291 systemically through subcutaneous injection. It will come packaged in a single-use vial typical for such drug products.
Concerns over bioavailability in systemic delivery of peptide drugs depend on the nature of the disease or medical conditions being treated, the mechanism of action (MoI) of a given drug, and the desired effect of the therapy. As of 2017, over 60 peptide drugs have been approved and over 150 other peptide drugs are in development, many of which are administered systemically. This is not an issue for ISP technology. The product has been administered in a number of animal models for different diseases and conditions, with consistently demonstrated biological activity and functional benefits.
The strong mechanistic data in preclinical animal models, potential for a well-tolerated safety profile, and opportunity to treat a life-threatening, severely debilitating condition with no treatment options are the basis for NervGen’s focus of early efforts on SCI. Notable ISP results and attributes from preclinical studies include
locomotive recovery with a significant subset of SCI animals achieving near complete recovery
100% of SCI animals experiencing partial to complete recovery of bladder function at higher doses
results reproduced in multiple studies, at multiple laboratories, and in multiple preclinical models, including several separate SCI studies.
ISP was found to be relatively simple and noninvasive to administer, producing lasting improvement in locomotive and bladder functions after a finite period of daily injections. It was administered during an extended time window post-SCI, which could facilitate conduct of clinical trials and suggests applicability to both acute (early) and chronic (long-term) nerve-damage patients.
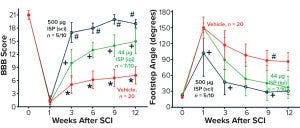
Figure 3: Sample preclinical data collected using the PTPσ inhibitor, ISP (8); measurement of rodent locomotion (seven weeks of daily treatment) (50–70% response rate).
As illustrated in Figure 3, the Baso, Beattie, Bresnahan (BBB) rating score and the foot-stepping angle (FSA) are objective locomotor measures commonly used to assess locomotor recovery in preclinical models. BBB scores of ~21 and FSA of <20° were recorded in rodents before injury. BBB scores of 1 and FSA of 150° were recorded one week after injury. After seven weeks of daily treatment with ISP, BBB scores of 15–19 and FSAs of 30–37° were observed at week 12 after injury. Compared with nontreated rodents, the treated animals had remarkable improvement in their BBB scores of 8–12 points and in FSAs of 49–56° at week 12. Equally important are the observed dose-dependent responses, with the higher 500-µg daily dose producing more improvement in BBB scores and FSAs. In that case, an average BBB score of 19 was observed among responding animals at week 12, nearly reaching the preinjury average BBB score of 21. All improvements in treated rodents were statistically significant compared with those receiving placebo, as well as when results were compared between the two doses. A response rate of 50–70% was observed. It should be noted that even though treatments were halted on day 49, improvements continued and were persistent to the end of the experiment on day 84.
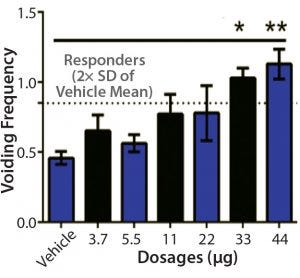
Figure 4: Sample bladder dose-response data (5)
Recovery of urinary bladder function is a critical issue in the management of paralyzed patients. Eliminating or reducing catheterization could reduce urinary tract infections, hospitalizations, morbidity, and overall healthcare costs for such patients. Improvement in bladder function was observed depending on dosage in a 2015 study (5). For the two groups receiving the highest doses of IPC, improvements were observed in all animals. Improvement in bladder function also was observed in a 2018 study (Figure 4).
A natural extension of NervGen’s ISP technology beyond SCI would be application to peripheral nerve injury (PNI), which also is largely caused by traumatic injury. ISP has been shown to promote regeneration of damaged nerves and improvement in function after root avulsion in a rodent model (7). Root-avulsion injury is a physical separation of a spinal nerve from the spinal cord that often occurs in violent events such as motor vehicle and sports accidents. In the rodents, the avulsed nerve root consists of a long segment of peripheral nerve and a small fragment of central nervous tissue. To restore peripheral motor function after avulsion, the injured neurons must regenerate through inhibitory scar tissue and enter into the peripheral nerve trunk to form synapses eventually with distal target muscles. Treatment with ISP increased the numbers of axons regenerating across scar tissue and enhanced motor functional recovery, indicating that such treatment could play a role in regenerating peripheral nerves and improve functional recovery.
Another demonstration of the potential of this technology to treat PNI was reported with a rodent model of dorsal root injury (9). Such injuries commonly result in loss of peripheral sensory function when peripheral nerves are unable to regenerate and cross the region between their dorsal root and the spinal cord. Treatment with ISP promoted peripheral nerve regeneration across that zone into rodent spinal cords, restoring sensory function.
Beyond Spinal Cord Injuries
In working to understand what controls the regrowth of axons after spinal cord injury, we also discovered several other medical conditions involving nerve injury in which scarring occurs and CSPGs affect axon regrowth. Axons that are severed in peripheral nerves, such as those found in the arms and legs, have a limited capacity to regrow. However, when a lesion is severe and closer to the torso, a CSPG-laden scar also can hinder recovery because damaged peripheral axons also upregulate the same PTPσ receptor.
My team and I showed that ISP can help heal injuries to peripheral nerves by speeding up the growth of injured nerves across and beyond the scar to the muscles they control.
Multiple sclerosis (MS) is an inflammatory-mediated disease of the nervous system that affects both the brain and the spinal cord. It damages myelin, the material that wraps and insulates nerve cells. Loss of myelin slows down or blocks electrical messages traveling between the brain and the rest of the body, causing physical symptoms such as tremors, cramping, weakness, and lack of coordination. CSPG-filled scar-like plaques that form in damaged areas play a critical role in preventing recovery by blocking migration of immature, potentially myelin-forming stem cells that exist in large numbers within the central nervous system. Using animal models, we found that ISP promotes stem-cell migration into these lesions, with return of the myelin sheath leading to functional recovery (10).
So it is possible to use the product in a combination therapy with stem cell treatments as well as other drugs. We believe that the MoI behind it can be synergistic and complementary to that of other therapies. The details behind such combinations will depend on the other therapies in question. So we look to evaluate combination therapy with our product as we advance our drug through development.
We also teamed up with investigators who study scars that form after heart attacks. When a heart attack occurs, sympathetic axons that control the heart rate are damaged in the vicinity of a forming scar. Just like in the spinal cord, those cut axons die back away from the lesion core, and their regenerating tips become entrapped within the outer edges of the scar, causing irregular heartbeats (arrhythmias) that can be lethal. In animal models of heart attack, systemic treatment with ISP has promoted new growth of damaged axons back into the scarred areas, stopping those arrhythmias (6).
Those results in rodents with infarcted hearts suggest that PTPσ inhibitors might be used to prevent sudden cardiac death caused by loss of heart function (sudden cardiac arrest). This is the largest cause of natural death in the United States, amounting to some 325,000 adult deaths there each year. It is estimated to be responsible for half of all deaths from heart disease (11).
In concert with NervGen Pharma, my team and I are creating innovative solutions for treatment of nerve damage. Together, we have identified an ISP analogue peptide (NVG-291) for treating human patients. It is our hope that this technology can improve the lives of many people living with debilitating nerve damage.
References
1 Kolata G. Rat Nerves Repaired and Rejoined With Spine. The New York Times 14 November 1987; https://www.nytimes.com/1987/11/14/us/rat-nerves-repaired-and-rejoined-with-spine.html.
2 Silver J, Miller JH. Regeneration Beyond the Glial Scar. Nature Rev. Neurosci. 5, 2004: 146–156; https://doi.org/10.1038/nrn1326.
3 Tom VJ, et al. Studies on the Development and Behavior of the Dystrophic Growth Cone, the Hallmark of Regeneration Failure, in an In Vitro Model of the Glial Scar and After Spinal Cord Injury. J. Neurosci. 24(29) 2004: 6531–6539; https://doi.org/10.1523/JNEUROSCI.0994-04.2004.
4 Shen Y, et al. PTPσ Is a Receptor for Chondroitin Sulfate Proteoglycan, an Inhibitor of Neural Regeneration. Science 326(5952) 2009: 592–596; https://dx.doi.org/10.1126%2Fscience.1178310.
5 Lang BT, et al. Modulation of the Proteoglycan Receptor PTPσ Promotes Recovery After Spinal Cord Injury. Nature 518(7539) 2015: 404–408; https://10.1038/nature13974.
6 Gardner R, et al. Targeting Protein Tyrosine Phosphatase σ After Myocardial Infarction Restores Cardiac Sympathetic Innervation and Prevents Arrhythmias. Nature Comm. 6(6235) 2015; https://10.1038/ncomms7235.
7 Li H, et al. Enhanced Regeneration and Functional Recovery After Spinal Root Avulsion by Manipulation of the Proteoglycan Receptor PTP σ. Sci. Reports 5(14923) 2015; https://10.1038/srep14923.
8 Rink S, et al. Recovery After Spinal Cord Injury by Modulation of the Proteoglycan Receptor PTP σ. Exp. Neurol. 309, 2018: 148–159; https://doi.org/10.1016/j.expneurol.2018.08.003.
9 Yao M, et al. Targeting Proteoglycan Receptor PTPσ Restores function After Spinal Cord Dorsal Root Injury by Activation of Erks/CREB Signaling Pathway. Neuropharmacol. 144 2019: 208–218; https://10.1016/j.neuropharm.2018.10.035.
10 Luo F, et al. Modulation of Proteoglycan Receptor PTPσ Enhances MMP-2 Activity to Promote Recovery from Multiple Sclerosis. Nature Comm. 9(4126) 2018; https://doi.org/10.1038/s41467-018-06505-6.
11 Sudden Cardiac Death (Sudden Cardiac Arrest). Cleveland Clinic: Cleveland, OH, 14 May 2019; https://my.clevelandclinic.org/health/diseases/17522-sudden-cardiac-death-sudden-cardiac-arrest.
Dr. Jerry Silver is coinventor and scientific advisor to NervGen Pharma, unit 1703, Three Bentall Centre, 595 Burrard St, Vancouver, BC V7X 1J1, Canada; 1-778-888-4101; www.nervgen.com; [email protected]. He is also professor of neurosciences at Case Western Reserve University’s School of Medicine and adjunct professor in the department of neurosurgery at the Cleveland Clinic Foundation.
You May Also Like





