Spray Freeze-Drying Technology: Enabling Flexibility of Supply Chain and Drug-Product Presentation for BiologicsSpray Freeze-Drying Technology: Enabling Flexibility of Supply Chain and Drug-Product Presentation for Biologics
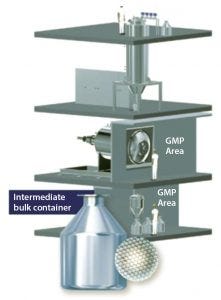
Figure 1: Production-scale equipment designed and manufactured by Meridion Technologies GmbH (12)
Biopharmaceutical drug substances (DSs) and drug products (DPs) commonly are stored frozen or refrigerated to maintain stability through long-term storage, handling, and transportation (1). Temperature excursions during storage and transport can affect product quality adversely by compromising the safety and efficacy of these molecules. Thus, cold-chain management throughout the shelf life of these products is a critical component in the supply chain strategy for them.
The cost and complexity of cold-chain management is a well-known challenge faced by the biopharmaceutical industry. Highly expensive and complex logistics can be attributed to several industry and regulatory-related factors, just a few of which are mentioned here. Adequate product storage equipment is required at all distribution nodes in the supply chain and must be qualified, maintained, and monitored continuously. Temperature-controlled transportation requires insulated shipping containers and/or other specialized packaging along with data loggers (2) and radiofrequency identification (RFID) tags to track and record product temperature histories (3). Frozen material transport is especially challenging for high-volume products, requiring large quantities of dry ice or other phase-change materials in each shipment pallet. Single-use, disposable bags that are commonly used for storage of frozen drug substances/products can pose a potential risk of failure from rupture and leakage due to stresses incurred during shipping and handling (4). So the cold supply chain is a challenging and expensive paradigm for biopharmaceuticals. It also is a potential obstacle to developing a robust supply chain into emerging markets and warmer climates.
One strategy to alleviate the challenges and risks associated with the cold chain is to manufacture biopharmaceutical drug substances as bulk powders. Removal of water from a DS is known to stabilize biologics, so lyophilization (freeze-drying) is a common process used in DP manufacturing. The potential for long-term, room-temperature stability in dry-powder form could facilitate commercial manufacturing and distribution into developing markets without the need for a complex cold chain infrastructure.
Another important advantage afforded by a bulk-powder DS strategy is the flexibility for multiple DP options throughout a product development lifecycle. In one scenario, multiple liquid DP presentations can be manufactured from a single bulk powder DS batch by varying the reconstitution volume to achieve a range of concentrations. In another scenario, sterile bulk-powder DS could be manufactured and then aseptically filled into vials, cartridges, dual-chambered syringes, or other device technologies for eventual use by patients/caregivers through reconstitution and administration. That could enable novel powder DP presentations for market differentiation that have the added benefits of long-term, room-temperature storage and patient convenience.
Conventional Technologies for Sterile Bulk-Powder Manufacturing: Manufacturing of bulk powders is not new to the biopharmaceutical industry. It can be accomplished using widely available processing methods such as freeze drying and spray drying. Both conventional processes offer advantages and present some challenges and limitations.
Conventional freeze drying, for example, is used commonly to produce biologics and other pharmaceuticals (e.g., small molecules and oligonucleotides) in bulk powders for single dose units or trays. The process requires batch processing and generally does not yield homogeneous, free-flowing powders. That is a potential drawback for high-volume biologics, as is the low processing efficiency and container-to-container variability that can affect product quality attributes (5, 6).
Similarly, spray drying is a well-established continuous manufacturing process for generating aseptic bulk powders from a liquid phase. This method is ideal for active pharmaceutical ingredients (APIs) and other low–molecular-weight (LMW) compounds but is not a suitable platform for all biomolecules — especially not for those susceptible to heat and shear stresses (7). Typical spray drying can yield fine powders with a mean particle size <10 µm. Fine particles are well suited for inhalation delivery (8), but they are challenging to fill–finish into single-dose units for parenteral delivery.
Evaluation of Spray Freeze-Drying Technology
We evaluated the feasibility of an alternative technology — spray freezing and dynamic bulk freeze drying (“spray freeze drying”) — using a monoclonal antibody (MAb) as a model biopharmaceutical drug substance and process equipment designed and manufactured by Meridion Technologies GmbH (Müllheim, Germany). As described herein, this technical evaluation was performed to determine the benefits and challenges of the technology for sterile bulk-powder biologics manufacturing.
Spray freeze-drying is a two-step, semicontinuous process that addresses the limitations of conventional technologies for biologics processing. The Meridion equipment integrates both process steps into a fully contained unit that is amenable to clean-in-place (CIP) and steam-in-place (SIP) processes (12). It enables aseptic production of a free-flowing bulk powder that is homogeneous and ready to fill into a number of container types.
Process and Equipment Overview: The first step in the process is spray-freezing. This operation is analogous to conventional spray-drying in that it is amenable to continuous processing for high-volume manufacturing. During this step, a liquid substrate is dispersed into individual droplets of similar size using low-shear frequency nozzles (12) and operated according to the controlled laminar-jet break-up principle. The droplets fall through a cooling tower that is supplied with liquid nitrogen to maintain its interior air temperature below –110 °C, which flash-freezes the droplets into discrete spheres. The diameter of individual spheres is controlled by the nozzle diameter that can range from 250 to 1,000 µm (12). The flexibility to produce larger droplet sizes with this technology also can be beneficial in terms of minimizing interfacial stresses during processing, representing another advantage over conventional spray-drying. Typical spray-drying processes yield smaller droplets sizes with higher interfacial surface areas, which can increase the potential for degradation at the air–liquid interface (9–11).
The second step in the spray freeze-drying process is dynamic bulk lyophilization.
During this operation, the frozen spheres are discharged into a temperature-controlled rotary dryer through an internal transfer tube. There, they are freeze-dried in bulk under a vacuum with constant, gentle mixing. Meridion’s rotatory dryers also contain an infrared heating element that provides additional energy for rapid drying. In this process, sublimation energy is transferred through both radiation and a temperature-controlled drum surface (12). When drying is complete, the lyophilized spheres are discharged into an intermediate vessel that can dock to a sterile isolator for powder filling. Figure 1 shows the Meridion production-scale setup.
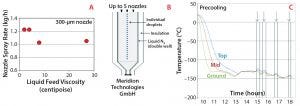
Figure 2: (A) Spray rates achieved for liquid monoclonal antibody (MAb) formulations of varying viscosities; (B) schematic of the cooling tower designed by Meridion Technologies (12); (C) temperature control inside the cooling tower during spray freezing
Production of a Bulk-Powder MAb DS: For evaluation of the spray-freezing step, liquid MAb formulations were prepared at concentrations of 50, 100, 150, and 200 g/L in the presence of a noncrystallizing sugar. Formulation viscosities were from 2 to 27 centipoise (cP). The spray-freezing operation used three spray nozzles, each with a 300-µm orifice. The nozzle frequency and liquid feed rate were controlled to ensure generation of discrete droplets. For the range of viscosities evaluated, spray rates were between 1.0 and 1.2 kg solution per hour per nozzle, with no significant reduction in throughput for the highest-viscosity formulation. Thermocouples positioned inside the cooling tower showed adequate temperature control during the process (Figure 2). Selection of a larger nozzle orifice (up to 400 µm) can be used to maximize throughput.

Figure 3: (A) Meridion LyoMotion30 pilot-scale rotary dryer (12) used for MAb bulk powder production; (B) representative temperature and pressure profiles generated during a dynamic bulk freeze-drying process; (C) bulk MAb powder with individual spheres of ~800 µm
For the MAb formulations described above, rapid drying times of 24–30 hours were achieved using Meridion laboratory-scale and pilot-scale rotary dryers (Figure 3). Bulk freeze-drying times for these MAb formulations — ranging 10–36% w/w total solids — were significantly faster than conventional freeze-drying because the product is in constant motion with this technology, and the entire particle surface is made available for heat and mass transfer. 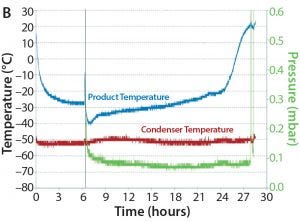 The critical process parameters (CPPs) include drum temperature, infrared (IR) heater power, and drum rotation speed. We chose those parameters to maximize process yields (>97%) and obtain bulk powders with a uniform size distribution and virtually no particle attrition. As for most freeze-dried products, low residual moisture is important for ensuring long-term product stability. Residual moisture levels of <1% were achieved for all MAb formulations processed using this technology.
The critical process parameters (CPPs) include drum temperature, infrared (IR) heater power, and drum rotation speed. We chose those parameters to maximize process yields (>97%) and obtain bulk powders with a uniform size distribution and virtually no particle attrition. As for most freeze-dried products, low residual moisture is important for ensuring long-term product stability. Residual moisture levels of <1% were achieved for all MAb formulations processed using this technology.
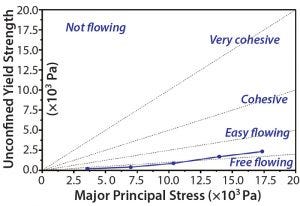
Figure 4: Flow-function test of a representative MAb powder batch using a shear cell apparatus; dotted lines guide the designation of powder flow behavior.
Bulk Powder Characterization
Key performance attributes of the MAb bulk powders produced using Meridion equipment include free-flowing powder quality, reproducible and narrow size distributions, rapid reconstitution, and long-term stability.
Free-Flowing Bulk Powder: We tested flow function of several MAb powder batches using a shear-cell apparatus, a method commonly used to characterize powder-flow behavior. Powders are classified
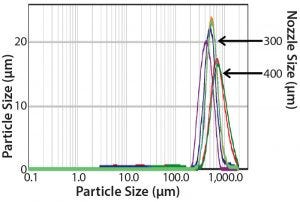
Figure 5: Particle-size distributions of MAb powder batches produced using Meridion laboratory-scale and pilot-scale rotary dryers
as either “free flowing,” “easy flowing,” “cohesive,” “very cohesive,” or “not flowing.”
Inside the shear cell,
powders are exposed to a range of consolidating stresses. Upon rotation of the cell, the sliding friction force is measured with a torque sensor and plotted as a function of consolidation stress. Based on the plots generated during our flow-function tests, the MAb powder batches produced by spray freeze drying are characterized as free flowing to easy flowing (Figure 4).

Table 1: Mean sphere size as a function of nozzle diameter
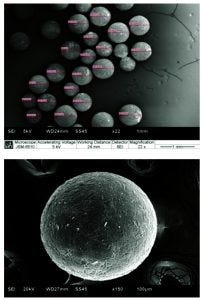
Figure 6 Scanning electron microscope (SEM) images of freeze-dried MAb formulation spheres
Reproducible Particle Size and Shape: We analyzed particle size by laser-light scattering for several laboratory-scale and pilot-scale MAb powder batches (Figure 5). The overlay shows narrow, reproducible size distributions with virtually no fines under 10 µm. Comparison of the size distributions obtained for two nozzle sizes — 300 µm and 400 µm — also shows that sphere size can be controlled during processing by selecting the desired nozzle orifice size (Table 1). In addition, scanning electron microscopy (SEM) imaging confirmed consistent generation of spherical shaped particles (Figure 6).

Figure 7: (A) SEM image of a freeze-dried sphere cross-section showing high porosity; (B) reconstitution of powdered MAb formulation using different volumes of water for injection (WFI) diluent; (C) MAb aggregation levels determined by size-exclusion chromatography (SEC) after reconstitution with different volumes of WFI diluent
Rapid Reconstitution: Rapid and flexible reconstitution is another advantage associated with bulk powders produced by spray freeze-drying technology. SEM images showing sphere cross-sections reveal the highly porous nature of these particles. Because of their high surface area and porosity, rapid wetting and dissolution of the MAb powders could be achieved with different volumes of water for injection (WFI) as a diluent (Figure 7). Complete reconstitution was achieved consistently with two to four minutes of gentle agitation regardless of sphere size. We used size-exclusion chromatography (SEC) to confirm that MAb aggregation levels remained unchanged after reconstitution and were not significantly affected by varying the reconstitution volume (Figure 7).
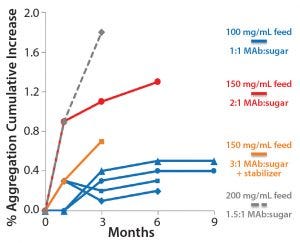
Figure 8: Room temperature (25 °C) stability of bulk-powder MAb formulations produced from liquid feed solutions of 100–200 mg/mL
Long-Term, Room-Temperature Stability: We found the stability of our MAb bulk powders produced using this technology to be highly formulation dependent. For conventional freeze-drying of biologics, lyoprotectants such as amorphous sugars usually are needed to provide protection from the stresses of drying and to enhance product stability in the freeze-dried state. Therefore, it is not surprising that the ratio of MAb to sugar component significantly affected bulk MAb powder stability (Figure 8). Another important factor in determining bulk-powder stability was the MAb concentration in the liquid feed solution. Overall, a MAb concentration of 100 g/L in minimal formulation containing sugar (1:1 w/w MAb:sugar) with a surfactant showed virtually no increase in aggregation over time at a 25 °C storage condition (Figure 8). Projections made from existing stability data suggest that long-term, room-temperature storage is feasible for this MAb product in spray freeze-dried form.
Additional Considerations
Based on preliminary feasibility studies, spray freeze-drying using Meridion technology shows promise as a semicontinuous processing method for producing biopharmaceutical DS bulk powders. However, full integration of this process technology for aseptic manufacturing involves some challenges and considerations. From technical, quality, and regulatory perspectives, the approach to aseptic process validation using process simulation (media filling) will require careful design and implementation. The powder collection and handling interface design and facility logistics also will require a significant amount of investigation from a validation standpoint. Suitable bulk-powder packaging technologies also should be identified to ensure sterility maintenance and other critical quality attributes (CQAs) during storage and transport. Finally, some facility-fit and engineering considerations will be related to the vertical height of the cooling tower and liquid nitrogen required.
Despite those challenges, spray freeze-drying appears to be a promising strategy for simplifying supply chain logistics. This offers a novel platform for room-temperature storage of biopharmaceuticals in bulk-powder form. An added advantage of this technology is its potential to enable drug-product innovation, providing flexible delivery presentations that could have added benefits of room-temperature storage and patient convenience.
Acknowledgments
We are grateful for collaboration with Drs. Bernhard Luy and Matthias Plitzko of Meridion Technology GmbH and for their contribution toward bulk-powder processing. We also thank Frederick Osei-Yeboah, Sarah Sundelacruz, Catherine Lynes, and Brandon Leveille at Biogen for their help with bulk-powder characterization.
References
1 Puri M, et al. Evaluating Freeze-Thaw Processes in Biopharmaceutical Development: Small-Scale Study Designs. BioProcess Int. 13(1) 2015: 34–45.
2 Kumar N, Ajeya J. Temperature Excursion Management: A Novel Approach of Quality System in Pharmaceutical Industry. Saudi Pharm. J. 25, 2017: 176–183.
3 Prakash G, Pravin Renold A, Venkatalakshmi B. RFID Based Mobile Cold Chain Management System for Warehousing. Procedia Engineering. 38, 2012: 964–969.
4 Goldstein A, et al. Freeze Bulk Bags: A Case Study in Disposables Implementation. BioPharm Int. 2 November 2009, supplement.
5 Rambhatla S, Tchessalov S, Pikal MJ. Heat and Mass Transfer Scale-Up Issues During Freeze-Drying, 3: Control and Characterization of Dryer Differences Via Operational Qualification Tests. AAPS PharmSciTech. 7(2) 2006: E61–E70.
6 Cook IA, Ward KR. Headspace Moisture Mapping and the Information That Can Be Gained About Freeze-Dried Materials and Processes. PDA J. Pharm. Sci. Technol. 65(5) 2011: 457–467.
7 Ahmad M, et al. The Challenge of Drying Method Selection for Protein Pharmaceuticals: Product Quality Implications. J. Pharm Sci. 96(8) 2007: 1886–1916.
8 Saleem K, Petkar S, Somavarapu S. Chapter 19: Rationale for Pulmonary Vaccine Delivery — Formulation and Device Considerations. Micro- and Nanotechnology in Vaccine Development. Skwarczynski M, Toth I, Eds. Elsevier: New York, NY, 2017: 357–371.
9 Costantino HR, et al. Protein Spray-Freeze Drying: Effect of Atomization Conditions on Particle Size and Stability. Pharm. Res. 17(11) 2000: 1374–1383.
10 Mumenthaler M, Hsu CC, Pearlman R. Feasibility Study on Spray-Drying Protein Pharmaceuticals: Recombinant Human Growth Hormone and Tissue-Type Plasminogen Activator. Pharm. Res. 11(1) 1994: 12–20.
11 Maa YF, Nguyen PA, Hsu SW. Spray-Drying of Air–Liquid Interface Sensitive Recombinant Human Growth Hormone. J. Pharm. Sci. 87(2) 1998: 152–159.
12 Luy B. Innovative Bulk Drying of Frozen Microspheres by Spray Freeze Drying. AAPS National Biotechnology Conference: Boston, MA, 17 May 2016.
Corresponding author Deirdre Lowe is a senior engineer; Mehak Mehta, PhD, and Geetha Govindan are scientists; and Kapil Gupta is director of protein pharmaceutical development at Biogen, 225 Binney Street, Cambridge, MA 02142; [email protected]; www.biogen.com.
You May Also Like






