A Multidisciplinary Approach to Manufacturing BiotherapeuticsA Multidisciplinary Approach to Manufacturing Biotherapeutics
https://bioprocessintl.com/wp-content/uploads/2015/03/032015-Lyons-Multidisciplinary.mp3
WWW.PHOTOS.COM
Optimizing antibody manufacturing processes has gone beyond the first-order goal of achieving elevated protein titers and now also focuses on understanding biologic and manufacturing process variables that define cellular machinery and protein quality. A holistic approach to biotherapeutic manufacturing incorporates several applied disciplines such as biology, engineering, process control, signal processing, and modeling to reduce the “black-box” model of cell- based protein production into an operational design space. This is in line with the US Food and Drug Administration’s quality by design initiative.
Ongoing work at Bend Research (a division of Capsugel Dosage Form Solutions) is focused on developing and implementing a process-development methodology that applies state-of-the- art automated sampling technology with on-line and at-line cell-state monitoring technologies — both novel and existing — to bioreactor processes. This methodology couples such tools with applied mathematics, data-integration techniques, and an understanding of bioreactor and biologic processes. Ultimately, this methodology will improve the tuning and managing of cell and protein production by optimizing bioreactor processes using real-time data-integration technologies. This is the purpose of process analytical technology (PAT) for supporting quality by design (QbD).
The goal of our work at Bend Research is to develop a solutions-based client offering focused on optimizing product quality while streamlining R&D, pilot- scale, and commercial process definition. In addition, we are using modeling technologies with a focus on optimizing or maintaining product quality across manufacturing sites and scales. The focus of internal and external research programs has been driven largely by existing and anticipated industry interests and challenges. Here we describe efforts to develop integrated biotherapeutic manufacturing hardware and software technologies to reduce risk and optimize our clients’ fed-batch and perfusion processes.
Process Effects on Cells and Proteins
Ongoing work in bioreactor process optimization is focused on understanding variables that affect bioreactor productivity, on developing predictive models around those variables, and on defining processes to improve bioreactor performance. Development of robust predictive models using key variables of interest requires extraction and rapid analysis of large quantities of bioreactor data during model manufacturing fed- batch or perfusion runs to capture cellular dynamic and steady-state performance data. Automated sampling hardware and data curation and storage tools can streamline that process.
Critical features that enable a model- driven approach to bioreactor process definition include on-line and at-line analytics and sampling technologies that allow for significantly larger and deeper data sets to be collected in real time. Bend Research has developed platform technologies such as the real-time Modular, Automated Sampling Technology (MAST) system and experimental design and data-reduction JUBiL software to increase the breadth and quality of data sets being generated in bioprocessing and associated analytical laboratories.
The MAST system aseptically draws samples from R&D, pilot-scale bioreactors and delivers those generated samples to analytical instruments such as the Nova BioProfile Flex bioanalyzer, liquid chromatography systems, or a Gilson sample collector. Our custom software tools integrate experimental design with data acquisition and aggregation and model development to streamline knowledge generation during and after bioreactor runs. Combining these hardware and software tools allows for more rapid and robust process development and optimization.
The Mast System
In implementing PAT, biopharmaceutical companies continually strive to gain fundamental understanding of the physical situation of cells within bioreactors and how processes affect those cells’ ability to deliver target product quality. Although implementation of on-line tools such as dielectric spectroscopy (1, 2) and Raman spectroscopy (3) can provide insight, a gap remains in integrating the data they produce with off-line measurements of such variables as cell density, viability, metabolite levels, and protein titers.
In collaboration with Pfizer and other major biopharmaceutical companies, we are working to advance the MAST platform toward a goal of providing a complete data-management solution. This system automatically supplies scheduled samples to analytical devices for near–real-time data collection that can be used to enhance process understanding — and ultimately improve or help automate bioreactor control. The system combines all capabilities needed to successfully collect and route bioreactor samples to analytical devices. It has been deployed in nine facilities so far, where it has been used to take thousands of aseptic samples.
With a pumping design that allows for transport of liquids that have very high solids content, the MAST system also solves problems associated with sampling from high–cell-density cultures. It overcomes sample-supply issues by incorporating a positive-displacement pump directly onto its sampling module. The pump pushes samples using hydrostatic pressure, which can prevent sample-integrity issues associated with use of a vacuum to draw samples. To prevent cell-debris accumulation, the system flushes and sanitizes all product- contact components after each sample is taken. Its valve stems and body are designed to minimize crevices and abrupt changes in line size, reducing areas for accumulation.
Studies at both Bend Research and Pfizer have repeatedly shown that results for samples automatically collected and analyzed on a Nova BioProfile Flex instrument are consistent with those collected and analyzed manually but can save one to two hours of labor each day. Figure 1 shows typical results that are equivalent for MAST and manual samples.
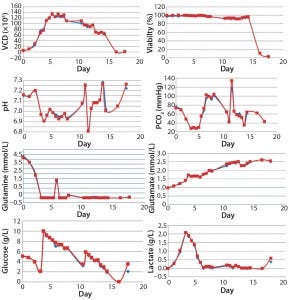
Figure 1: Comparing automated MAST samples (blue) with those manually collected (red) and analyzed for viable cell density (VCD), viability, pH, and PCO2
Recently the MAST system has been expanded to enable simultaneous sampling from up to eight bioreactors and distribution of those samples to as many as four analytical instruments or sample-collection destinations. The same technologies of the bioreactor sampling modules have been incorporated into the new hardware components. Data destinations have included the Nova BioProfile Flex bioanalyzer, a manual sample-collection station designed by Bend Research, and a Gilson liquid- handling system. We are developing integration programs for Roche’s Cedex HiRes and BioHT analyzers and Beckman Coulter’s ViCell system, as well as several ultraperformance liquid chromatography (UPLC) and liquid-chromatography–mass spectroscopy (LC-MS) analyzers.
Bioreactor tests have demonstrated that the MAST system provides automated, reliable, and contamination- free sampling that is comparable with manual samples. This scale-independent sampling system provides for frequent sampling, enabling more intensive process-control schemes. Savings can be achieved in labor and process optimization/efficiency. Highly efficient Sample Pilot modules also have potential use with disposable systems and downstream applications.
The combination of automated sampling and advanced on-line measurement with at-line analysis (e.g., dielectric and Raman spectroscopy) creates a synergistic environment for accelerated process understanding. This emerging process-development methodology shows promise for shortening development timelines and delivering higher-quality processes that will significantly reduce biopharmaceutical cost of goods.
Software
We have had the opportunity to work with a broad range of clients in the biologics industry. A common theme running throughout this industry concerns the increased number of data sets generated during R&D, pilot-scale, and current good manufacturing practice (CGMP) development and manufacturing — with a need for seamless transfer of those data sets among disparate environments. And a key challenge faced in PAT implementation is integrating off- line and on-line data streams to analyze the data in real time.
Bend Research has developed an automated data-aggregation program to rapidly query, analyze, and visualize cell- culture data. JUBiL (dJango Unified Biologics Laboratory) software is a web- based suite of data management tools developed to be flexible, extensible, and scalable for implementation in innovative and dynamic cell-culture R&D laboratories. It automatically aggregates data from a host of bioanalytical devices such as Aber Instruments’ dielectric spectroscopy system and those mentioned above. Additionally, the program collects metadata, user comments, and observations associated with cell-culture experiments to enable filtering and word-search querying of its databases. JUBiL data analysis features include
seamless electronic access to valuable information
facile data annotation for documenting comments and observations
automated real-time data plotting
automated time-normalization to enable run-to-run comparisons.
Figure 2 shows JUBiL examples comparing aggregated cell density and dielectric spectroscopy capacitance measurements. This illustrates the benefits of automated data aggregation, visualization, and analysis systems to the increasing focus on data interpretation. Such presentations render data easily retrievable for verification and in-depth analysis, enabling real-time process troubleshooting and optimization. Automated data aggregation is a gateway to more complex first-principle and multivariate analyses: e.g., completing mass balances for metabolites or carbon dioxide, compiling mixing and mass- transfer data to verify outputs from computational fluid dynamics (CFD) simulations, or generating principal components analysis (PCA) or partial least squares (PLS) models of cell-culture performance.
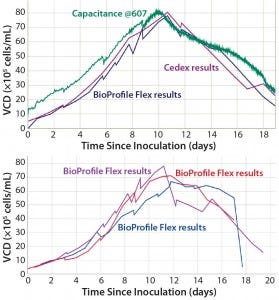
Figure 2: JUBiL screenshots illustrate automated data plotting (top) and run-to-run comparison features (bottom).
Combining MAST-enabled offline analytics with online analytical tools and JUBiL data analysis enables PAT and improves time-course resolution of cell- culture dynamics. Increased resolution of such dynamics is crucial for improving product-development and biomanufacturing life cycles — from developing robust processes that produce high-quality biologics to implementing process control that delivers optimal product every time.
Demonstration Facilities
To capitalize on the combination of integrated hardware and software platforms and to take advantage of the bioprocess science, our company has invested in a suite of 2-L Broadley-James bioreactors equipped with DeltaV controllers from Emerson Process Management in a demonstration laboratory. It is available for demonstration work with clients who seek to transfer MAST equipment into their own laboratories and for process development and optimization of clients that do not have in-house facilities.
We have taken an integrated approach toward characterizing and understanding biomanufacturing processes across scales. A critical component to optimum use of a MAST system with on-line data collection (e.g., using dielectric spectroscopy and Raman spectroscopy) is an ability to aggregate and visualize data in real time. We believe that a rational approach to data generation and reduction — integrating biological and engineering fundamentals with data handling and reduction to practice — can lessen risk and streamline drug development. In addition, parallel software and modeling development creates an integrated laboratory environment for optimization of biologics development and manufacturing.
Real-Time By Design
As the biotherapeutics industry moves beyond a “black-box” model of cell-based protein production, process development efforts are focused on understanding biologic and manufacturing process variables that define protein quality. Integrated approaches to biotherapeutic manufacturing — incorporating applied disciplines such as biology, engineering, process control, signal processing, and modeling — are needed to develop an operational design space.
Bend Research has collaborated with its pharmaceutical partners to develop new technologies that encompass critical features of this novel approach to bioreactor process definition. Automated, aseptic sampling technologies and new spectroscopic techniques allow collection of large data sets, which are subsequently analyzed using software that facilitates real-time data visualization and aggregation. These enhanced tools offer process-development and optimization capabilities the industry demands for streamlining and accelerating biomanufacturing.
References
1 Ansorge S, Esteban G, Schmid G. Multifrequency Permittivity Measurements Enable On-Line Monitoring of Changes in Intracellular Conductivity Due to Nutrient Limitations During Batch Cultivations of CHO Cells. Biotechnol. Prog. 26, 2010: 272–278.
2 Braasch K, et al. The Changing Dielectric Properties of CHO Cells Can Be Used to Determine Early Apoptotic Events in a Bioprocess. Biotechnol. Bioeng. November, 2013: 1–13.
3 Teixeira AP, et al. Advances in On-Line Monitoring and Control of Mammalian Cell Cultures: Supporting the PAT Initiative. Biotechnol. Adv. 27, 2009: 726–732.
Corresponding author Jeffrey Breit is director of inhalation technology; Brandon Downey is a research chemical engineer; Clint Pepper is director; Amber Broadbent is director of applied math, modeling, and computational science; and David Lyon is senior vice president of research at Bend Research (a division of Capsugel Dosage Form Solutions), 64550 Research Road, Bend, OR 97701; 1-541-382-4100, fax 1-541-382-2713; [email protected]; www. bendresearch.com.
You May Also Like






