Therapeutic Gene Editing: Tools to Facilitate Basic Science or Stimuli for a Paradigm Shift in Biomanufacturing?Therapeutic Gene Editing: Tools to Facilitate Basic Science or Stimuli for a Paradigm Shift in Biomanufacturing?
Historically, fundamental science and process engineering were separated by distinct vernaculars and a decade or more in the translation pathway of candidate therapeutics from laboratory to bedside (1). This crude metric holds true for the origins of the modern pharmaceutical industry, namely fine chemicals that supported the high-margin small molecules that constitute the majority of the pharmacopoeia even today. But as illustrated by deeply interwoven careers, companies, and technologies — including those related to monoclonal antibodies (MAbs) — that classic gap between science and bioprocessing is rapidly closing (2). Arguably, therapeutic gene editing technologies could represent the greatest stimuli yet to span this chasm conclusively and build networks for future emerging technologies.
Therefore, the raison d’etre of this article is simple: to urge the global bioprocessing community to recognize therapeutic gene editing as not simply a scientific curiosity to adorn the ephemeral pages of Nature, Science, and Cell — but rather, the means for making a profound change to bioprocessing. Thus there is a potential change for which we are not in all cases fully prepared.
Introduction To Therapeutic Gene Editing
A number of discoveries have contributed to significant changes in quality-of-life throughout the ages: e.g., fire, wheels, iron, automobiles, computers, printed circuits, the Internet, social networks, and smart phones. With such “disruptive innovations,” technology progresses quickly, creating new paradigms that are taken for granted by following generations without the need to understand what came before. These forever alter the technology landscape (3).
The challenge with new technologies is to parse correctly those that are merely hype from the select few that will be truly revolutionary (4–6). Today we find ourselves in the midst of a new set of biological discoveries with the potential to help usher in a new age of medicine — a genetic revolution. With the advent of technologies such as DNA sequencing that have outpaced Moore’s Law (7), the future of medicine is progressing as rapidly as revolutions before. That will lead to technologies for identifying disease, developing personal cures, and using our own cells to stimulate therapeutic and regenerative capacity that could revolutionize health and wellness for future generations.
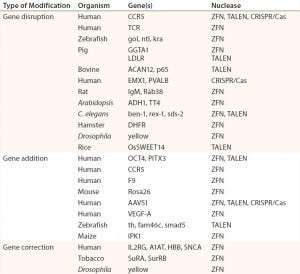
Table 1: Abbreviated list of examples of ZFN-, TALEN-, and CRISPR/Cas-mediated genome editing in human cells and model organisms; references for each available in cited source (11)
Gene Editing Technologies
This new age in biology is founded on decades of science leading up to this point. Previous discoveries, government expenditures, experimental successes, and clinical failures all have contributed to the current state of therapeutic gene editing and led to the discovery of the clustered regulatory interspaced short palindromic repeat (CRISPR) and CRISPR-associated gene 9 (Cas9) system (8). Early gene therapy attempts were halted when poor understanding led to unanticipated nonrandom integration, which caused deadly immune responses and leukemia (9, 10). Patient safety concerns and caution prevailed, hobbling the industry’s progress at the turn of the millennium and forcing a lengthy period of introspection. During that time, biology and technology caught up with the promise to give insight into the causes of on-target effects and routes to mitigating off-target risks for future therapeutics.
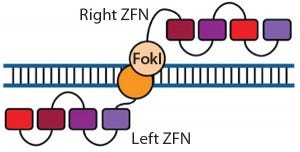
Figure 1: Zinc-finger nucleases (ZFNs) are fusions of the nonspecific DNA cleavage domain from the FokI restriction endonuclease with zinc-finger proteins. ZFN dimers induce targeted DNA double-strand breaks (DSBs) that stimulate DNA damage-response pathways. The binding specificity of the designed zincfinger domain directs the ZFN to a specific genomic site (11, 19).
In response, emerging technologies have been developed, with each generation gaining efficiency and specificity (11). These include zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and now CRISPRs, which are showing promise for therapeutic use. All three technologies edit the genome of treated cells by hunting out target sequences and replacing or mutating their genetic code.
Complex genomes often have many identical or closely homologous sequences that can pose targeting challenges (11). So efforts must be made to limit unwanted interactions and ensure expected therapeutic effects as well as possible. Each technology offers means to combat off-target effects including creation of heterodimers and engineering of active complexes themselves (11, 12).
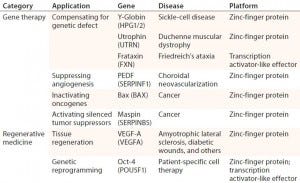
Table 2: Examples of therapeutic applications for engineered transcriptional activators; references for each available in cited source (11)
Potential Clinical Implications
One exciting prospect for genome-editing technologies is their ability to correct the root cause of a disease rather than offer only palliative care for its symptoms (11). ZFN clinical results are the most accessible and reported so far. ZFN approaches have been used to treat monogenic disorders, such as sickle-cell disease (Table 2). They have been targeted at the CCR5 locus for human immunodeficiency virus (HIV) (13). And they have been used to enhance immunotherapeutics by suppressing expression of endogenous T-cell receptor (TCR) genes, potentially leading to allogeneic treatments (11, 14).
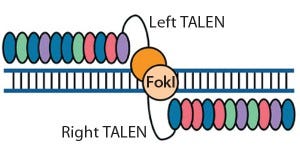
Figure 2: Transcription activator-like effector (TALE) nucleases are fusions of the FokI cleavage domain and DNA-binding domains derived from TALE proteins. TALEs contain multiple 33–35 amino acid repeat domains, each of which recognizes a single base pair. Like ZFNs, TALENs induce targeted DSBs that activate DNA damage-response pathways and enable custom alterations (11, 19).
Although some ZFNs can permeate cell membranes, most editing platforms are delivered through ex vivo electroporation or packaged into adenoviral, adenoassociated virus (AAV), or lentiviral vectors. Each such vector accommodates different cassette sizes for insertion. It has been found for TALENs that adenoviral vectors more successfully transfer intact genes than lentiviral vectors do (15).
Commercial Viability
As with all clinically active proteins/ antibodies, manufacturing systems that build in current good manufacturing practices (CGMPs) are required for maintaining patient safety and mitigate risk. Additionally, safety testing and large-scale manufacturing of therapeutics using gene-editing technologies — including good genomic practices (GGPs) (16) — need to be performed at a price point that will allow the therapy to show pharmacoeconomic benefit that will enable its cost to be reimbursed by insurance companies.
Current tools and technologies for manufacturing and component testing can be used to bring gene-editing tools to CGMP standards. Implementation of commercial production systems is relatively straightforward but not trivial. The challenge will be to understand enough about the way the ZFN, TALEN, or CRISPR edits target genes and whether they can be designed for safety. That includes improving product specificity and limiting off-target effects.
Recent improvements in reduction of off-target effects have been developed through the use of tools such as X-ray crystallography to determine the structure of activated Cas9 molecules responsible for cutting DNA. Subsequent “high-fidelity” CRISPRs can be designed by making modifications to the Cas9 protein that increase its specificity and reduce off-target DNA cuts, leading to mutations. Using technologies that limit DNA cutting has also brought the potential for engineering an epigenome by forming interfering (CRISPR-I) or activating (CRISPR-A) forms (17).
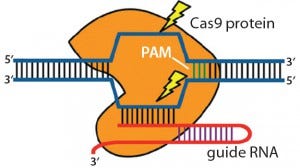
Figure 3: Clustered regulatory interspaced short palindromic repeats (CRISPRs) are loci that contain multiple short, direct, base-pair repeats and provide acquired immunity to bacteria and archaea. CRISPR systems rely on CRISPR ribunucleic acid (crRNA) and transactivating crRNA (tracrRNA) for sequencespecific silencing of invading foreign DNA. In type II of the three CRISPR/Cas systems, Cas9 serves as an RNA-guided DNA endonuclease that cleaves DNA upon crRNA-tracrRNA target recognition (11, 19).
Intellectual property will play a role in future selection of technologies for clinical development. For example, the three companies currently developing programs for commercial use of CRISPR/Cas9 (Editas Medicine, CRISPR Tx, and Intellia) will need to make significant legal decisions related to how to protect their specific technologies. Uncertainty related to licensing and the ability to use technologies for commercial use has not appeared to inhibit relations between those CRISPR companies and therapeutic developers, including Novartis, Bayer, Juno, Vertex, and Genethon (18).
Finally, delivery systems will need to be developed and produced under CGMP conditions. These might include AAV or lentivirus-based systems that need to be selected based on their ability to fit the gene-editing systems within them. Cas9 produced by Staphylococcus aureus bacteria was discovered by Feng Zhang at the Broad Institute. It is much smaller than Cas9 typically used by researchers and produced by Streptococcus pyogenes, allowing the system to be packaged into a CRISPR–AAV construct and delivered systemically (20). That led to Duke University researchers’ demonstration of successful delivery of the CRISPR-Cas9 system in a Duchenne muscular dystrophy mouse model, thus treating a genetic disease in a fully developed living mammal (11).
The Genetic Revolution
The question remains whether geneediting technologies have what it takes to start a revolution. Although uses such as reduced immunogenicity of gene-modified chimeric antigen receptor T (CAR-T) cell therapies show promise, mainstream interest and current large investments warn of irrational exuberance. Yet if tools and technologies allow us to further elucidate the Cas9 structure, for example, and modify it to increase specificity, mitigate off target effects, and better use AAV to deliver the Cas9 payload, then this might just be the perfect storm of a disruptive healthcare technology.
Only time will tell. There is assuredly a “critical mass” and developmental acceleration fueled by data, investment, and potential clinical applications, suggesting that we are on the brink of an impending revolution. One thing is for certain: Gene modification, cell therapy, and tissue engineering all will play a significantly influential role in the future of regenerative medicine. These advances in genetic engineering will benefit greatly the quality of human life for many generations to come.
References
1 Cooksey D. A Review of UK Health Research Funding. HMSO: Norwich, UK, 2006.
2 Hitt E. Melding Talents with a Career in Bioprocessing. Science 15 July 2011; doi:10.1126/science.opms.r1100105.
3 Christensen C. The Innovator’s Dilemma: When New Technologies Cause Great Firms to Fail. Harvard Business Review Press: Cambridge, MA, 1997.
4 Baker M. Caution and Hope for the Stem Cell Industry. Nat. Rep. Stem Cells 7 May 2009; doi:10.1038/stemcells.2009.66.
5 Russell AJ. The End of the Beginning for Tissue Engineering. Lancet 383, 2014: 193– 195.
6 Lysaght MJ, Hazlehurst AL. Tissue Engineering: The End of the Beginning. Tissue Eng. 10, 2004: 309–320.
7 Hayden EC. Technology: The $1,000 Genome. Nature 507, 2014: 294–295; doi:10.1038/507294a.
8 Sander JD, Joung JK. CRISPR-Cas Systems for Editing, Regulating and Targeting Genomes. Nat. Biotechnol. 32, 2014: 347–355.
9 Jenks S. Gene Therapy Death: Everyone Has to Share in the Guilt. J. Natl. Cancer Inst. 92, 2000: 98–100.
10 Ramos CA, Dotti G. Chimeric Antigen Receptor (CAR)-Engineered Lymphocytes for Cancer Therapy. Exp. Opin. Biol. Ther. 11, 2011: 855–873.
11 Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, and CRISPR/Cas-Based Methods for Genome Engineering. Trends Biotechnol. 31(7) 2013: 397–405.
12 Szczepek M, et al. Structure-Based Redesign of the Dimerization Interface Reduces the Toxicity of Zinc-Finger Nucleases. Nat. Biotechnol. 25(7) 2007: 786–793.
13 Tebas P, et al. Gene Editing of CCR5 in Autologous CD4 T Cells of Persons Infected with HIV. New Eng. J. Med. 370(10) 2014: 901–910.
14 Torikai H, et al. A Foundation for Universal T-Cell Based Immunotherapy: T Cells Engineered to Express a CD19Specific Chimeric-Antigen-Receptor and Eliminate Expression of Endogenous TCR. Blood 119(24) 2012: 5697–5705.
15 Holkers M, et al. Differential Integrity of TALE Nuclease Genes Following Adenoviral and Lentiviral Vector Gene Transfer into Human Cells. Nucl. Acids Res. 41(5) 2013: e63; doi:10.1093/nar/gks1446.
16 Barker RW, Brindley DA, Schuh A. Establish Good Genomic Practice to Guide Medicine Forward. Nat. Med. 19, 2013: 530; doi:10.1038/nm0513-530.
17 Jiang F, et al. Structures of a CRISPRCas9 R-Loop Complex Primed for DNA Cleavage. Science 351(6275) 2016: 867–871; doi:10.1126/science.aad8282.
18 Rood J. Who Owns CRISPR? The Scientist 3 April 2015; www.the-scientist.com/?articles.view/articleNo/42595/title/Who-Owns-CRISPR.
19 Gersbach CA, Gaj T, Barbas CF. Comparing Genome Editing Technologies. Gen. Eng. News. 34(5) 2014; 1, 32–34; doi:10.1089/gen.34.05.02.
20 Ran FA, et al. In Vivo Genome Editing Using Staphylococcus aureus Cas9. Nature 520(7546) 2015: 186–191; doi:10.1038/nature14299.
Further Reading
Harding A. CRISPR: The Path to Clinical Trials. Xconomy 29 January 2016; www.xconomy.com/boston/2016/01/29/crispr-the-path-to-clinical-trials.
Kingery K. CRISPR Treats Genetic Disorder in Adult Mammal. Duke Today 31 December 2015; http://today.duke.edu/2015/12/crisprmousedmd.
McGreevey S. High-Fidelity CRISPR. HMS News 6 January 2016; http://hms.harvard.edu/news/high-fidelity-crispr#.VpPZCsWctd4.twitter.
News Release: High-Fidelity CRISPR-Cas9 Nucleases Have No Detectable Off-Target Mutations. Massachusetts General Hospital: Boston, MA, 6 January 2016; www.massgeneral.org/about/pressrelease.aspx?id=1883.
Press Release: Généthon and CRISPR Therapeutics announce Research Collaboration. CRISPR Therapeutics: Basel, Switzerland, 16 December 2015; http://crisprtx.com/news-events/news-events-press-releases-2015-12-18.php.
Press Release: Bayer and CRISPR Therapeutics AG Join Forces to Discover, Develop and Commercialize Potential Cures for Serious Genetic Diseases. CRISPR Therapeutics: Basel, Switzerland, 21 December 2015; http://crisprtx.com/news-events/news-events-press-releases-2015-12-21.php.
Eric Roos is strategic alliances leader for cell therapy, and Rebecca Buzzeo is global commercial director for cell biology at Thermo Fisher Scientific. Kim Bure is director of regenerative medicine at Sartorius Stedim (Göttingen, Germany). James Smith is a CASMI research associate and professor in the University of Oxford’s Nuffield Department of Orthopedics, Rheumatology and Musculoskeletal Sciences. And corresponding author David Brindley is founder and academic director of CASMI’s Translational Stem Cell Consortium, senior healthcare translation research fellow in the medical sciences division’s department of pediatrics at the University of Oxford, Cooksey-Saïd fellow in healthcare translation at the University of Oxford’s Saïd Business School, honorary senior research associate at the University College London School of Pharmacy’s Centre for Behavioral Medicine (London, UK), a research fellow in cell therapy commercialization at the Harvard Stem Cell Institute, and regenerative medicine regulation and risk management lead at the USCF-Stanford Center of Excellence in Regulatory Science and Innovation (CERSI) in Stanford, CA; [email protected].
You May Also Like





