Automation in Process DevelopmentAutomation in Process Development

Figure 1:
Overcoming the bottleneck is a key statement often heard in the context of bioprocessing. Acceleration of both up- and downstream processes have therefore become a general need in the biopharmaceutical industry. Not only because this will release resources to comply with the increasing numbers of drug candidates, but also because this will shorten the time-to-market, giving more time to realize a return on investment before the patent expires.
Thus there is a need to apply new innovative methods to accelerate process development. This article describes how automation in the different areas of process development can contribute to achieving these targets and presents an overview of the applications currently in use on Tecan Freedom EVO® robotic workstations:
Cell line development*
Cell culture upscaling
Antibody development*
Downstream process development
Process analytical technology (PAT).
(*Application is not described in this article. For more information, please refer to the Tecan contact at the end of this article.)
Upstream Process Development
A generic framework for rapid identification and optimization of factors affecting soluble protein yield in microplate fermentations as a prelude to the predictive and reliable scale-up of optimized culture conditions has been applied on Freedom EVO. The application of design of experiments (DoE) approaches reduced the total number of experiments necessary, whereas experimentation at the microplate scale increased experimental throughput and reduced costs.
Along with biological parameters, well geometry, shaking speed, and liquid fill volume were included as factors in the DoE design because they strongly influence oxygen transfer into the wells. Consideration and analysis of the model results showed six of the original 10 variables to be important at the screening stage (three after optimization). The latter included the microplate shaking speed, indicating oxygen transfer rate as a key basis for scale-up. Compared with standard reference conditions, both the screening and optimization designs gave up to threefold increases in the soluble protein yield (a ninefold increase overall). The optimization process predicted a distinct optimum set of conditions for protein expression, which could be verified experimentally at larger scale (Figure 1).

Figure 1: ()
Downstream Process Development
Tecan Freedom EVO workstations can be used for fully automated determination of protein adsorption isotherms using GE Healthcare PreDictor™ plates. For these experiments, the ratio between protein bound to chromatographic medium and protein liquid concentration must be varied within a certain interval. Dedicated PreDictor plates with controlled chromatography media volumes of 2–50 µL may be used, requiring only the preparation of one protein solution. The same volume of protein solution is added to all wells and incubated while mixing for at least three hours. Initially, at time zero all protein will be present in liquid phase (Figure 2). At equilibrium (after three hours or more), the liquid concentration has decreased, and protein has been adsorbed on the chromatographic medium, which is given as capacity (Q solid phase). The relationship between protein concentration in liquid at start and at equilibrium equals the phase/volume ratio (Vliquid/Vchrom medium = β), illustrated as dotted lines in Figure 2.
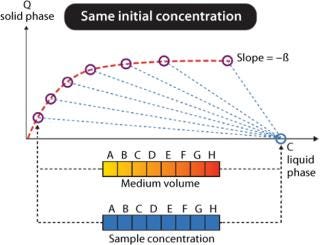
Figure 2: ()
Process Analytical Technology
The newly introduced RoboColumns®, automation compatible, miniaturized chromatographic columns in a 96-array SBS format, are in use for many industrial applications including rapid in-process monitoring of monoclonal antibodies from production-scale fermentation broth (Figure 3). For this purpose, samples from fermentation were loaded onto a row of eight RoboColumn 5-5 (100 µL CV) packed with ProSep®-vA High Capacity affinity media. After a short rinsing step, the bound antibodies were rapidly eluted by an acidic buffer (the first dimension), immediately neutralized in the receiver plate by the robotic platform, and submitted to high-performance cation-exchange chromatography (the second dimension). Figure 3 shows the resulting elution profile. Automation accelerated the time-consuming sample preparation step for this analysis by nearly one order of magnitude and enabled data traceability.

Figure 3: ()
Summary
The combination of Freedom EVO robotic workstations and screening procedures has the potential to shorten development time for biopharmaceutical production significantly and may contribute to overcoming future bottlenecks. Modules for downstream processing include options for simple batch-adsorption techniques, high-throughput techniques (PreDictor plates from GE Healthcare), resin-filled tips, and miniaturized chromatographic columns (RoboColumns from Atoll).
This scientific instrumentation is not for use in human clinical or diagnostic procedures. PreDictor is a trademark of GE Healthcare companies; Freedom EVO is a registered trademark of Tecan, Te-Shake is a trademark of Tecan, RoboColumn is a trademark of Atoll, ProSep is a trademark of Millipore. Data in Figure 2 and picture kindly provided by © 2008 GE Healthcare (all rights reserved).
REFERENCES
1.) Islam, R. 2007. Framework for the Rapid Optimization of Soluble Protein Expression in Escherichia coli. Biotechnol. Prog. 23:785-793.
You May Also Like






