A friend of mine was returning home from a vacation in Rome this week, and her flight from Europe to Newark was delayed and diverted because someone’s child had measles on the intended connecting plane. Measles! Someone thought it was okay to spread that around.
Often as we put together a themed issue — whether focused on a manufacturing pillar or something new like this month — a subtheme seems to just happen. This month, thanks to authors from Biogen and MilliporeSigma along with the drug-product contribution from CBX, that subtheme turned out to be vaccines. Combined with my friend’s experience, this has turned my thoughts to the antivax movement and the damage done. What began as a mere public-relations problem for the biopharmaceutical industry has become a growing economic and public health disaster.
It traces back to a flawed study by a now-disgraced scientist who claimed a connection between one adjuvant and autism. Thanks to confirmation bias, that was all it took to convince anyone who may have ...
Looking Back, Looking Ahead
Check both ways before proceeding — always good advice. Industry analysts at GlobalData and Mergermarket (an Acuris company) have reviewed 2017 and made predictions for the biopharmaceutical industry in 2018. Set against a backdrop of remarkable advances in technology and the growing relevance of biosimilars, last year was marked by both disappointments and noteworthy innovations.
GlobalData points to major regulatory approvals in “nearly every major therapeutic area.” These include CAR T-cell oncology treatments, the first biologic for atopic dermatitis, and the first drug for primary progressive multiple sclerosis. Global director of therapy analysis and epidemiology Claire Herman says, ‘‘Each fills a critical clinical unmet need and alters the treatment paradigm.’’ But R&D saw significant setbacks in 2017 as well, including two candidate failures for Alzheimer’s disease and two major approved drugs that did not meet endpoints in key oncology trials.
Meanwhile, biosimilars ar...
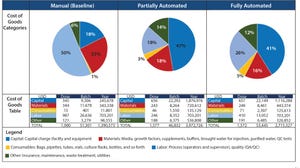
Figure 1: Impact of team head count and labor annual costs for operations and quality personnel on the overall cost of goods (CoG) per batch
In part 2, we continue to analyze manufacturing costs of an autologous cell therapy. A typical process involves the expansion and activation of cells derived from a single patient, which is currently very labor-intensive. To date, there is little published information on overall production costs (
1
). In
part 1
, we used a software modeling platform to identify opportunities for potential cost savings. We developed a baseline model of a cell therapy manufacturing process using the production of autologous dendritic cells for immunotherapy. Here we discuss opportunities for cost reduction and costs associated with implementing automation. We also explore strategies for increasing throughput to meet rising demand through the analysis of net present cost (NPC).
Opportunities for Cost Reduction
Labor:
Based on our cost of goods (CoG) analysis, an area that appears to...
ADOBESTOCK
Bioprocesses traditionally use (fed-)batch cell culture processes for production of recombinant proteins and therapeutics. In batch bioprocessing, material flow is discrete, with a hold step between two unit operations, and product is harvested only once for each unit operation. Batch processes have been studied extensively and optimized through numerous advancements in experimental design (
1
,
2
), monitoring (
3
–
5
), measurement techniques (
6
–
9
), and control strategies (
10
–
12
). However, such processes require large facility footprints for equipment (
13
) as well as sterilization, load, and harvest steps between individual batches, which in turn decreases their overall space–time yield (STY) (
14
). Furthermore, many traditional batch processes have shown inconsistent performance in productivity and critical quality attributes (CQAs). Therefore, companies increasingly turn to alternative operation modes for overcoming those process bottlenecks associated with batch culture.
With t...
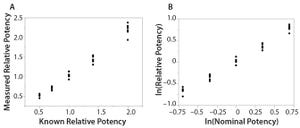
Figure 1: Simple linear regression analyses using original data and logarithm base e transformed data; (LEFT) bivariate fit of measured relative potency by nominal potency; (RIGHT) bivariate fit of ln(relative potency) by ln(nominal potency)
Analytical linearity along with assessments of precision and accuracy determine the range for bioassays (
1
). Practitioners can include coefficient of determination (
R
2
) criteria from a linearity study in the bioassay validation protocol. Herein I illustrate the relationship of
R
2
to study design and analytical method variation.
Overview of the Simple Linear Regression Model
Dilutional linearity assesses the “ability (within a given range) of a bioassay to obtain measured relative potencies that are directly proportional to the true relative potency of the samples” (
1
). With bioassay data, a logarithm base e data transformation provides the appropriate use of a simple linear regression model by reducing the impact of variance heterogeneity with increasing pot...
Photo 1: Twin-column equipment Contichrom CUBE Combined (BENCHTOP SCALE, LEFT) and EcoPrime Twin (PILOT SCALE, RIGHT)
Periodic countercurrent (PCC) processes increasingly are being evaluated as alternatives to single-column batch capture processes. Some of the advantages of PCC processes over single-column processes include shortening of processing time and/or reduction of required resin volume through increased productivity; reduction in resin costs through improved resin capacity use; and reduction in buffer consumption through increased column loading. Those advantages, however, come with increased equipment complexity and hardware costs. PCC processes and systems with two to up 16 columns of the same type have been proposed. As the number of columns increases, a process becomes more complex, potentially adding pumps, detectors, valves, and plumbing. The PCC process investigated in this study was a CaptureSMB process: a PCC process using two columns and modulated feed flow rates. All PCC processes are ...
GEA WESTFALIA (WWW.GEA.COM)
Clarification is typically the first unit operation in the purification of monoclonal antibodies (MAbs) and other proteins from mammalian cell cultures. This process removes cells and cellular debris from the culture fluid to produce a clarified cell culture supernatant that will be suitable for further purification (
1
).
Before 2000, depth and tangential-flow microfiltration were standard clarification technologies in the biopharmaceutical industry. Process-scale centrifugation was considered to be a significant capital investment, and bioprocessors had limited ability to control (minimizing) the effects of shear on mammalian cells. The following decade saw an industry-wide adoption of centrifugation with traditional periodic discharge of solids for primary clarification of mammalian cell cultures. That shift to centrifugation was facilitated by the need to scale processes up to handle 10,000-L to 15,000-L bioreactors, with increasing harvest cell culture densities and titers...
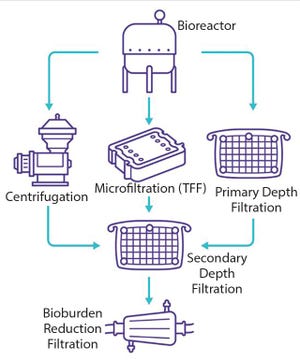
Figure 1: Usual clarification options for viral vaccines and vectors
Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (
1
). Viral vaccines are formulated with or without adjuvants and categorized as shown in Table 1.
Table 1: Classification of viral vaccines
Viral vaccine manufacturing processes can be templated. They follow a general scheme, starting with production in either an embryonated egg or mammalian/insect cell culture. After production, the bulk harvest material is processed to purify a vaccine of interest. Following upstream production and lysis (o...
Single-use systems used in drug-substance and drug-product process steps require higher assurance of container–closure integrity (CCI). (WWW.SARTORIUS.COM)
Identifying the greatest defect size, both for liquid leaks and microbial ingress, is a fundamental step toward protecting the integrity of single-use systems (SUS) under real process conditions. Integrity testing of such systems may become a prerequisite in the future because they are used in the most critical process steps, with detection limits correlating to liquid leaks and microbial ingress. Such testing guarantees the sterility of drug substances and drug products packaged in single-use systems and, therefore, enhance patient safety.
Single-use fluid-management solutions are being applied in critical downstream and final filling applications — including those that require container–closure integrity testing. Single-use bags constitute a core technology within those fluid-management solutions.
Biomanufacturers increasingly use such bags in critic...
The future success of biopharmaceutical businesses will depend at least partly on their ability to create meaningful brand experiences from the start of a drug program. By “brand,” I don’t mean logos and taglines. I’m talking about meaningfully unique experiences that directly affect clinical and patient needs — specifically, to address the growing demand for self-administered injectable therapeutics.
Whether you are a biosimilar developer trying to carve out differentiated value or a market leader looking at your patent protection in the rearview mirror, brand experience can improve your ability to capture market share effectively and deliver your life-enhancing molecule to the people who need it. After all, when a drug is subject to clinical parity among its competitors, its value comes from factors that increase the likelihood of each patient recipient’s administrating that therapy the right way, at the right time, every time.
Consumer experience and patient adherence are growing concerns. Consider the...
Sponsored Content
Chinese hamster ovary (CHO) cells are being pushed to their productivity limits in drug development and biomanufacturing. Biopharmaceutical companies working on promising new therapies increasingly struggle with development challenges such as weak protein expression, low-yield purification steps, and poorly optimized analytical techniques. To address those challenges, innovators need robust cell-expression platforms and advanced process, analytical, and biomanufacturing technologies.
Since 2012, KBI Biopharma has performed development and/or manufacturing services using more than 20 different cell lines generated by Selexis SA. In 2017, the two companies joined under the same parent organization: JSR Corporation. Stewart McNaull is senior vice president of business development for KBI.
McNaull’s Presentation
In 2016, over half of the development of manufacturing projects KBI worked on were for monoclonal antibody (MAb) products. In 2017, work expanded with other modalities such as bispecific and Fc-fusio...