Ensuring the Integrity of Single-Use Containers: Providing Robustness, Science, and Helium-Based Technology with a Detection Limit of 2 μmEnsuring the Integrity of Single-Use Containers: Providing Robustness, Science, and Helium-Based Technology with a Detection Limit of 2 μm
Sponsored Content

Single-use systems used in drug-substance and drug-product process steps require higher assurance of container–closure integrity (CCI). (WWW.SARTORIUS.COM)
Identifying the greatest defect size, both for liquid leaks and microbial ingress, is a fundamental step toward protecting the integrity of single-use systems (SUS) under real process conditions. Integrity testing of such systems may become a prerequisite in the future because they are used in the most critical process steps, with detection limits correlating to liquid leaks and microbial ingress. Such testing guarantees the sterility of drug substances and drug products packaged in single-use systems and, therefore, enhance patient safety.
Single-use fluid-management solutions are being applied in critical downstream and final filling applications — including those that require container–closure integrity testing. Single-use bags constitute a core technology within those fluid-management solutions.
Biomanufacturers increasingly use such bags in critical process applications such as for storage, mixing, shipping, and freezing of drug substances and drug products in current good manufacturing practice (CGMP) commercial production. Bag integrity failures can significantly compromise patient and operator safety, drug availability, and costs.
The biopharmaceutical industry has a poor understanding overall of the maximum defect sizes that will not cause liquid leaks and microbial ingress in single-use systems under real process conditions. Existing integrity testing methods often are not correlated to the leak size of concern and are inadequate for safety and regulatory compliance considerations. Feedback from regulators has stressed that manufacturers need to develop a physical integrity test method to validate the integrity of packaging at the supplier and postshipping and postinstallation at a customer site (1). Applying physical integrity test methods to every single bag used in production can overcome the statistical uncertainty of probabilistic testing approaches such as bacterial challenge testing. A physical test should be correlated to the greatest defect size that does not allow microbial ingress and/or a liquid leak.
This article describes how robustness and closure integrity can be achieved by design. It will give readers the first understanding of the science of container−closure integrity and introduce the Sartorius helium-based supplier integrity test, which can detect a defect size down to 2 μm as a proof of single-use system integrity.
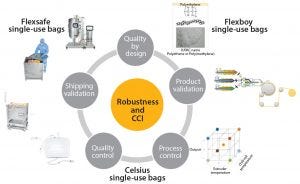
Figure 1: Robustness and closure integrity by design
Key Elements for Proven Integrity of SUS
Before implementation of quality by design (QbD), a consistent and reliable integrity-control strategy based on an initial risk analysis must ensure the inherent and consistent robustness of an SUS. The factors that can compromise integrity under real conditions of use and the understanding of mechanisms of liquid leakage — as well as their correlation with microbial ingress — are essential to defining, scientifically, the appropriate detection thresholds. In addition to visual inspection, appropriate and validated physical test methods then can be implemented, both at supplier and end-user sites to cover different types of potential failure modes. Operator training and support of standard operating procedures (SOPs) significantly improve handling of an SUS throughout its life cycle. In summary, SUS system integrity assurance can be improved in the following ways:
Understand market requirements and anticipate emerging regulatory requirements
Continuously improve product robustness based on QbD, stringent product validation, and extensive process and quality controls (Figure 1)
Apply good science to understand film behavior and determine the maximum allowable leakage limit (MALL, the greatest tolerable leak size that poses no risk to product safety) (2) for both liquid flow and microbial ingress under various process conditions.
Develop integrity testing technologies to detect the respective defect size of concern in all parts of complete SUS assemblies at both the supplier and the end-user facilities
Implement manufacturing infrastructure to test SUS assemblies at the supplier facility.
Robustness and Closure Integrity by Design
Knowledge of potential failure modes at each stage in a single-use system life cycle allows application of a systematic QbD approach to the development of single-use technologies.
Sartorius Stedim Biotech (SSB) has developed a film extrusion design space with associated manufacturing controls to guarantee consistent quality for Flexsafe, Flexboy, and Celsius single-use bags. To achieve this, we first defined critical quality attributes (CQA) and critical process parameters (CPP), which were the inputs for a full factorial design of experiments (DOE) performed with 23 experiments, eight variables, and three center points.
Process qualification, product packaging, and shipping validation allow us to ensure mechanical and microbial integrity of complete single-use assemblies. Many test methods, including tensile strength, seal strength, and burst tests, have been used to qualify the robustness of the films, components, bag chamber manufacturing processes, and complete bag assemblies.
Stringent shipping validation on filled single-use bags according to ASTM D4169 (3) — with real-life and laboratory tests as well as using a QbD approach — can establish robustness within an entire design space. For instance, a self-deploying bag design installed in its container can reduce the risk of integrity loss.
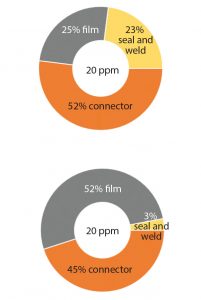
Figure 2: The main three root causes of the leaks on 2D bags are a leak on the film surface of the bag, a channel leak in the bag seal and in the weld of the port, and leaks at connections. The contribution of each of cause is shown as percentages at the manufacturing site (TOP) and at the end-user site (BOTTOM).
To implement the most efficient control strategy (e.g., film- and seal-strength integrity and routine microbial ingress testing according to ISO15747) (4), we use our quality risk management leaks observed at end-user sites from problems originating from our production facilities have three main root causes (Figure 2 TOP).
Seal, weld, and film failure modes are tested at 100% using a bag chamber leak test with detection limits of 40–100 μm, depending on bag chamber volume, whereas the connection for each individual component and combination is qualified and not tested at 100%.
Once the SUS arrives at the end-user facility, performing a nondestructive point-of-use leak test of all single-use bags to be used in critical process steps (in addition to operator training and handling procedures) prevents the risk of losing high-value product and enhances patient and operator safety. It ensures detection of the damage that may have occurred during shipping, storage, and handling of single-use bags at the end-user site.
SSB has performed a risk assessment that identifies a low probability of introducing a defect smaller than 100 μm– 200 μm during transport, handling, and storage of empty bags. Most leaks observed at customer sites are introduced into the film when the packaging is opened with scissors or knives or when connections are subjected to rough handling (Figure 2 BOTTOM).
The Science of Container–Closure Integrity
To develop a physical integrity test method with detection limits that can be correlated to liquid leaks and microbial ingress, it is crucial to understand the bacterial penetration and liquid flow mechanisms through components and materials used for single-use systems. In previous studies of different container systems, researchers tried different methods to establish and measure the respective MALL for liquid leaks and microbial ingress. Most of those studies were performed for rigid containers (5–10); however, some attempts have been made to study the defect size of flexible systems with physical tests (11–14). A review of literature on MALL shows a close relation between liquid leaks and microbial ingress (7–10). Liquid leaks and microbial ingress both are affected by process conditions, liquid attributes, and defect size.
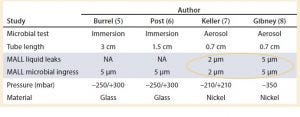
Table 1: A summary of past studies done in the past for different systems and at different pressures using microbial immersion and aerosol tests; and the correlation of the results with a liquid leak test; MALL = maximum acceptable liquid leaks
The results of those studies confirmed that a necessary condition for microbial growth is the presence of liquid in the defect pathway. But the probabilistic character of microbial growth and the presence of liquid in the defect pathway do not guarantee contamination of the container (9, 10). Table 1 lists the leakage sizes that do not pose a risk to product sterility under various conditions (type of bacterial challenge and conditions, microorganism, type of artificial leak and container).
For a rigid microtube, a formula can be used to calculate the threshold pressure at which liquid begins to flow through a defect (7, 8, 15). But little data are available on flexible packaging for which the ratio between channel lengths to leak diameter is very small. However, a microtube can be used to simulate the effects of bad weldings along flexible bag chambers or tube sealings.
On the other hand, environmental conditions such as pressure and temperature influence the propensity for single-use systems to leak. The greatest allowable defect size without occurrence of a leak or contamination depends on liquid surface tension, time, temperature, and measurement test method.
To study the integrity of flexible bags, Sartorius initiated a study program based on liquid leaks and on microbial growth with the films used for the production of Flexboy, Celsius, and Flexsafe SUS. The methods used, the results, and their interpretation are the subject of a publication in progress.
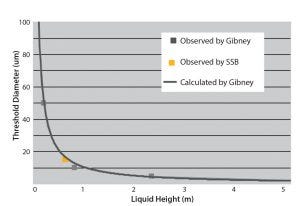
Figure 3: Liquid leakage and hydrostatic pressure for deionized water; the solid line shows calculated data by Gibney (8); GRAY boxes = experimental data points observed by Gibney, and the ORANGE box = the data point obtained by Sartorius Stedim Biotech.
The first results obtained by Sartorius about liquid leaks and microbial ingress with ethylene vinyl acetate (EVA S71) and polyethylene (PE S80) films of 300-μm and 400-μm thickness are compatible with what was examined in previous studies for rigid containers (8). Figure 3 shows the liquid leaks observed in our studies at a pressure of 70 mbar. This is consistent with the observation from 7-mm micro tubes at different pressures (8). 70 mbar is the hydrostatic pressure at the bottom of a 500-L bag or a 20-L bag for hanging (used for storage). Figure 3 shows that for the bags equal to 500 L in a storage condition, the leak size with no risk to sterility is 15 μm.
However, during shipment from one location to another by plane or truck, bags experience a range of different pressure and gravity conditions. The differential pressure generated during a transport was established by Post at –250/+300 mbar (6). In addition, our shipping validation shows that in the worst case, a bag can experience acceleration during transport by airplane or truck of 20g. According to the formula (7), the higher the pressure, the smaller the size of a defect that leads to a leak. Therefore, for a bag under a pressure above 200 mbar, the defect size that will allow a leak decreases to 5 μm. For a bag being transported by airplane, truck, or boat that experiences forces of 20g, the defect size that does not present a risk for the product is estimated to be around 2 μm.
Table 2: A series of microbial tests on polyethylene (PE S80) and ethylene vinyl acetate (EVA S71) films for leak sizes between 0 and 100 μm at atmospheric pressure; numerators show the number of samples that have bacterial ingress, and the denominator shows the maximum tests done for each defect size
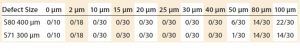
Table 2: A series of microbial tests on polyethylene (PE S80) and ethylene vinyl acetate (EVA S71) films for leak sizes between 0 and 100 μm at atmospheric pressure; numerators show the number of samples that have bacterial ingress, and the denominator shows the maximum tests done for each defect size
To make a correlation between liquid and microbiological methods, a series of microbial tests was carried out on PE S80 and EVA S71 films. At atmospheric pressure, no bacterial growth was reported up to a defect size of 40 μm (Table 2). The equivalent tests with microtubes show that bacterial growth starts to appear when the defect size ranges from 20 μm to 50 μm (7).
Because of the random nature of microbiological phenomena, microbiological integrity methods are probabilistic (2). That makes them unsuitable for system checks that may be necessary at our production site or at an end-user’s. Nevertheless, these methods are proven to be decisive when it comes to defining the maximum size of a leak that does not pose a risk for product sterility.
On the other hand, deterministic or physical test methods make it possible to obtain quantifiable, reproducible results with clearly defined and predictable detection limits. These detections can be correlated with the MALL previously defined by microbiological methods.
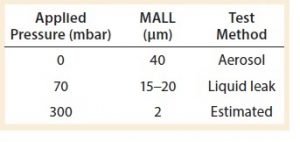
Table 3: The maximum allowable leakage limit (MALL) obtained or estimated so far by Sartorius at different applied pressures
Table 3 summarizes what has been established so far as the MALL either by liquid leak test or by microbial ingress test in single-use systems.
A Helium-Based Supplier Integrity Test (SIT)
The MALL of 2 μm estimated from our liquid leak and microbiological tests inspired us to develop and offer a physical, nondestructive integrity test method. With this new test, defects of 2 μm are detectable, so the test can be used to confirm the microbial-barrier properties of a single-use system.

Figure 4: Test system with two test chambers
Helium gas tracer technology is recommended in USP <1207> (2) as the most sensitive method currently available to identify a leak at the detection limit of 2 μm for SUS with volumes >1 L. In a helium-based integrity test, the container is placed in a sealed vacuum test chamber and connected to a helium filling line (Figure 4). The vacuum chamber ensures that no air or other gases can enter or exit during the test because that could interfere with the detection of helium. A mass spectrometer is used to measure the rate of increase in the concentration of helium that passes through the defects in the single-use system.
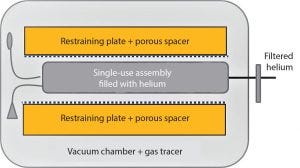
Figure 5: Schematic of test chamber design
A patented integrity test (16), including a special pumping technique, reduces the stress on the bag assembly by reducing the internal pressure of the bag along with the external chamber pressure. The test chamber is equipped with restraining plates and porous spacers to provide mechanical support for the assembly during the test and prevent masking effects caused by direct contact between the surface of the film and the stainless steel plate (Figure 5). This mechanical support allows testing with small inflation volumes and higher test gas pressures.
A pressure-sensing gross leak check prevents saturation of the test chamber with large quantities of helium and, therefore, practically eliminates system down times due to helium pollution. Once the vacuum in the test chamber has been created, helium is injected into the bag assembly and held for a fixed test time. During this time, the helium leak rate is measured with a mass spectrometer and compared against a preestablished acceptance criteria to make the pass–fail evaluation. The performance of testing equipment is verified routinely with a calibrated helium master leak.
Design-specific characteristics of SUS (such as the numbers of connections, the surface area, or materials of construction) can affect the leak rate. It is important, therefore, to determine the helium leak-rate acceptance criteria against a baseline of conforming products and confirm it by measuring the leak rates from defective assemblies.
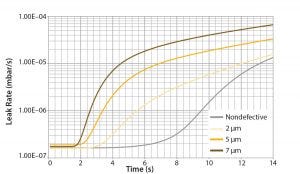
Figure 6: Leak rates of conforming and nonconforming bag assemblies
Permeation of helium through a bag film surface and tubing material can increase the helium concentration in the chamber significantly during the test, as shown by the “nondefective” curve in Figure 6. This phenomenon limits the ability of the test to detect defects that will cause leaks. However, because the increase in helium concentration caused by permeation occurs after increases caused by defects, it is possible to limit the test time and differentiate conforming from nonconforming bag assemblies with the target defect size. The pass–fail evaluation of the test must be executed within seconds of helium filling to prevent false-negative results due to gas permeation.
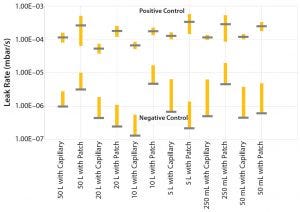
Figure 7: Leakage rates with six sigma confidence interval between defective and nondefective samples
Sartorius selected this technology for nondestructive testing of finished products for all SUS intended for use in commercial manufacturing of drug substances and drug products. A comprehensive validation study with over 700 tests performed on a large number of product types has shown that the design of a single-use system has a strong impact on the permeation rate of conforming products. Different types of defects such as pinholes in a bag film surface, channel leaks in the welding, or tube-to-hose-barb connections were used to represent different failure modes. A statistically significant number of 32 conforming and nonconforming samples per design/volume confirmed the capability of the validated method to detect 2-μm pinhole leaks (patch) as well as 20-μm channel leaks (capillary) with a confidence interval of at least six sigma between defective and nondefective samples (Figure 7).
The reproducibility and precision of our validated method allows users of SUS in critical process steps to reduce risks associated with introducing a defective consumable into their processes, jeopardizing the sterility of the systems.
Ensuring Product Sterility and Patient Safety
A consistent and reliable integrity control strategy must be based on an initial risk analysis before implementation of QbD to ensure the intrinsic (and consistent) robustness of single-use systems at all stages of a manufacturing process. In-depth knowledge of the factors that may compromise their integrity under real conditions of use and the understanding of mechanisms of liquid leakage (as well as their correlation with microbial penetration) are essential to a scientific definition of appropriate detection thresholds with regard to associated risks of loss of integrity. Appropriate and validated physical test methods and protocols then can be implemented at supplier and end-user sites to cover different types of potential failures in support of visual inspection and best practices.
The critical size of permissible leakage to ensure integrity of a single-use system will depend on the process step in which it will be used and the constraints it will have to bear (pressure, shocks, contact time, and so on). As of today, the only physical test method able to detect the maximum allowable leakage tolerable in a single use system used in any process conditions is the helium-based gas tracer that can detect down to a 2 μm defect. That detection limit correlates to liquid leaks and microbial ingress to ensure the best levels of product sterility and patient safety.
References
1 FDA–ASTM Workshop on Standards for Manufacture of Pharmaceutical and Biopharmaceutical Products Single Use Systems (SUS): A Microbiology Product Quality Perspective. Patricia F. Hughes, Branch Chief (Acting) CDER/OPQ/OPF/DMA; 11 October 2016.
2 USP <1207> Sterile Product Packaging-Integrity Evaluation. United States Pharmacopeia (USP); www.usp.org.
3 ASTM D4169-16: Standard Practice for Performance Testing of Shipping Containers and Systems. ASTM International, 2016; www.astm.org.
4 International Organization for Standardization. ISO15747: Plastics Containers for Intravenous Injection (First Edition), 2003; www.iso.org.
5 Burell L, et al. Development of a Dye Ingress Method to Assess Container–Closure Integrity: Correlation to Microbial Ingress. PDA J. Pharm. Sci. Tech. 54(6) 2000: 449–455.
6 Post E. Container Closure Integrity Test (CCIT): Cross-Validation of the Microbiological vs a Physico-chemical CCIT. PDA Europe Conference. Berlin, 2011.
7 Keller S. Determination of the Leak Size Critical to Package Sterility Maintenance. PhD Dissertation, Virginia Polytechnic Institute State University, VA, 1998.
8 Gibney M. Predicting Package Defects: Quantification of Critical Leak Size. Thesis (Master of Science in Food Science and Technology). Faculty of Virginia Polytechnic Institute and State University, 2000.
9 Kirsch L et al. Pharmaceutical Container/Closure Integrity II: The Relationship Between Microbial Ingress and Helium Leak Rates in Rubber-Stoppered Glass Vials. PDA J. Pharm. Sci. Technol. 51(5) 1997: 195–202.
10 Morton D, Lordi N, Ambrosio TJ. Quantitative and Mechanistic Measurements of Parenteral Vial Container/Closure Integrity: Leakage Quantification. J. Parenter. Sci. Technol. 43(2) 1989: 88–97.
11 Gilchrist JE, et al. Leak Detection in Flexible Retort Pouches. J. Food Prot. 52(6) 1989: 412–415.
12 Miyako Y, Tai H, Saitoh I. Helium Leak Test for Sterility Assurance of a Sealed Bag I: Relationship of Helium Leak and Pinhole Diameter. PDA J. Pharm. Sci. Tech. 56(4) 2002: 183–191.
13 Keller SW et al. Bioaerosol Exposure Method for Package Integrity Testing. J. Food Prot. 59(7) 1996: 768–771.
14 Pethe V, Dove M, Terentiev A. Integrity Testing of Flexible Containers. BioPharm Int. 24(11) 2011: 42–49.
15 Keller S, et al. Application of Fluid Modeling to Determine Threshold Leak Size for Liquid Foods. J. Food Prot. 66(7) 2003: 1260–1268.
16 Hogreve M. Sartorius Stedim Biotech GmbH, Deutsches Patent und Markenamt (German Patent and Trademark Office) DE102014013522B4. 30 March 2017.
Corresponding author Marc Hogreve is senior scientist of Integrity Testing, and Dr. Saeedeh Aliaskarisohi is a junior scientist at Sartorius Stedim Biotech GmbH, Goettingen, Germany; marc. [email protected]; 49-551-308-3752. Carole Langlois, senior product manager of Fluid Management Technology (FMT) and Marie-Christine Menier, compliance and regulatory affairs manager, are at Sartorius Stedim biotech GmbH, Aubagne, France.
You May Also Like






