WWW.COPPERHOUNDPICTURES.COM
As I write, the world is in a state of medical and economic uncertainty related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the coronavirus disease (Covid-19) that it causes. Our company group, Informa Connect, has been forced to reschedule some major conferences — including BPI West and BPI Europe — but it’s not alone. Many life-science meetings have been canceled or postponed, and some Biogen employees probably wish their management meeting late in February had been as well. That company has become infamous for an outbreak, and many organizations are asking employees who can do so to work from home. Others have suspended international business travel and conference attendance. The Pharmaceutical Research and Manufacturers of America (PhRMA) temporarily closed its offices after a visitor tested positive.
The biopharmaceutical industry usually thinks of pandemic preparedness and infectious-disease outbreaks as potential business opportunities. In fact, I...
https://stock.adobe.com
Even more so than cancer, Alzheimer’s disease is one disease that many people fear greatly. The thought of falling prey to the inevitable desecration of our own minds is something that makes even the bravest among us shudder. If we’re robbed of our sense of who we really are, we imagine, then we are doomed to live our last days without the dignity that defines us and that we hold most dear. The ultimate horror of the condition is that it is as indiscriminate, merciless, and devastating as a wind-swept wildfire.
So it is not surprising that a disease-modifying treatment for the condition has become a Holy Grail of sorts in the biotechnology industry. Alzheimer’s disease casts a shadow over many families. It seems that everyone is touched by it in one way or another. And it exacts a devastating financial toll on society, with Alzheimer’s patients needing around-the-clock care for an average of eight years. Some suffer for as long as 20 years.
The estimated cost for caring for America...
www.stock.adobe.com
Training departments in biopharmaceutical manufacturing facilities are at the forefront of ensuring that their employees are trained in accordance with regulatory compliance standards that govern the industry. More important, the purpose of training is to equip employees with relevant knowledge, skills, and abilities to perform their job functions competently. A competency-based curriculum has the potential to facilitate a training approach that addresses both the practical training needs and desired performance outcomes in a workplace.
Competency-Based Curriculum
Recently, I was involved in a curriculum review and redesign project at a pharmaceutical manufacturing organization. Early in the project, we agreed on a strategic objective that would see us align training curriculum with the organizational goals and performance outcomes. The redesign process led to an integrated compliance and competency-based curriculum framework that addressed both the regulatory and skills needs in the o...
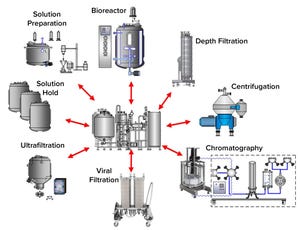
Figure 1: Clean-in-place (CIP) skid shared among multiple CIP clients; a shared CIP skid is shown with typical CIP clients of a monoclonal antibody process.
Risk of viral contamination is a an accepted part of developing biopharmaceutical products derived from mammalian-cell culture. Viral safety is achieved through a combination of complementary approaches such as selecting non–animal-derived raw materials, testing cell banks, testing for adventitious virus contamination during cultivation, and demonstrating viral reduction capacity of a purification process (
1
). The latter commonly is referred to as viral clearance by orthogonal purification.
Clearly, viral clearance and appropriate viral segregation are important considerations in biopharmaceutical manufacturing process and facility design. Good manufacturing practice (GMP) guidelines from the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) emphasize that appropriate segregation of process operations is a regulatory expectat...
Immunofluorescence image of BVDV (CP7 type); viral nuclei are stained blue; replication complexes are marked red by NS3 protein-binding antibodies.
Bovine serum products such as fetal bovine serum (FBS) are critical, nutrient-rich supplements frequently used in cell culture systems for a number of applications, including biotechnology, animal and human pharmaceutical and diagnostic manufacturing, and life-science research. Serum can be contaminated with adventitious agents that could increase its risk for use in cell culture systems. Bovine viral diarrhea virus (BVDV) is one of the most significant infectious diseases in the livestock industry worldwide because of its high prevalence, strong persistence, and severe clinical consequences (
1
). BVDV also is considered to be one of the most significant potential contaminants in mammalian cell culture because of its ability to reproduce in cells without causing notable cytopathic effects. Serum collection, manufacturing, and treatment processes must be rigor...
Exosomes are a subject of rapidly growing therapeutic interest in the biopharmaceutical industry for two principal reasons. The first reason is that they are the primary communicators of instructions from source cells to target cells. Exosome surface features define their destination. They recognize complementary features on target cells, dock with them, and deliver their programmed instructions in the form of microRNA. The second reason is that exosomes are immunologically silent. As normal human cell products, and by contrast with gene therapy vectors such as virus particles, exosomes bypass the issue of triggering an immune response that might interfere with therapy.
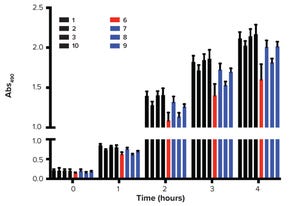
Figure 1: Chromatin structure and nucleosome array distribution in mammalian cell culture harvest (cch); agarose electrophoresis of Chinese hamster ovary cch is shown on the right. Each band in the cch corresponds to a nucleosomal array. The numerals at far right indicate the number of nucleosomes in each array. Note that the number of bas...
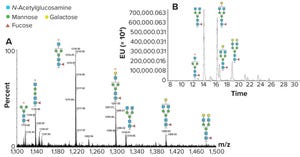
Figure 1: N-glycan analysis using peptide mapping and fluorescent tagging; (a) shows peptide-mapping signals observed for an IgG glycopeptide, and the region containing doubly charged signals is shown (only the glycans are shown for simplicity). A relatively high abundance of truncated glycan species can be seen (marked by an asterisk). (b) is a chromatographic profile of fluorescently tagged released N-glycans. The truncated species seen in Figure 1a are either absent or significantly reduced, indicating they come from in-source fragmentation processes in the peptide mapping experiment.
Both the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) have developed regulatory guidelines on biosimilars (
1, 2
). These comprehensive documents provide clear guidance on what the agencies expect from structural characterization studies. Using state-of- the-art instrumentation and techniques is expected, but the use of orthogonal techniques in structural comparability assessments is also requ...
Figure 1: PCR products gel electrophoresis; no viral nucleic acid was detected. The first spot on each panel (left corner) is the DNA ladder, and the bottom row on C shows the positive controls. Left to right are BTV, BCV, PRRSV, CSFV, PCV2, BP, BPV, PPV, ASFV, SVDV, and BHV. Smallest fragments in every spot are primer dimers.
Serum is the most commonly used supplement in cell culture. Fetal bovine serum (FBS) is the common choice because it contains high concentrations of growth factors and other important signaling molecules (e.g., adhesion proteins, nutrients, carrier proteins, cytokines, and hormones) required for cell survival and differentiation together with its buffering capabilities.
FBS production begins with the collection of whole blood from bovine fetuses under aseptic conditions. Once collected, the blood is allowed to clot, and the serum is mechanically separated. Raw serum is kept under sterile conditions and low temperature. Further processing such as filtration and irradiation typically ...
Biomanufacturing is constantly evolving, developing new treatments and therapies through cutting-edge methodologies and facing increasing integration of big data and analytics. But keeping up with innovation in this segment requires a workforce with advanced skill sets. The US State of Rhode Island (RI), with a rich manufacturing history and a thriving biotechnology sector, has taken on that challenge. We are preparing a robust talent pool — the greater Providence area contains over 1.6 million people — to become the highly skilled workforce that RI companies need. We’ve invested over US$40 million in workforce-development efforts since 2015.
Rather than relying on past approaches to workforce development, however, RI has spearheaded an innovative, demand-driven initiative to ensure that employees are trained to do exactly what companies are looking for. If businesses need employees with certain skills or knowledge, eventually market forces will generate the necessary supply. But that can take months or o...