BVDV Risk Mitigation: Dealing with Bovine Viral Diarrhea Virus in SerumBVDV Risk Mitigation: Dealing with Bovine Viral Diarrhea Virus in Serum
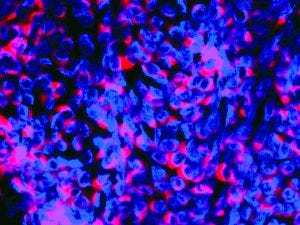
Immunofluorescence image of BVDV (CP7 type); viral nuclei are stained blue; replication complexes are marked red by NS3 protein-binding antibodies.
Bovine serum products such as fetal bovine serum (FBS) are critical, nutrient-rich supplements frequently used in cell culture systems for a number of applications, including biotechnology, animal and human pharmaceutical and diagnostic manufacturing, and life-science research. Serum can be contaminated with adventitious agents that could increase its risk for use in cell culture systems. Bovine viral diarrhea virus (BVDV) is one of the most significant infectious diseases in the livestock industry worldwide because of its high prevalence, strong persistence, and severe clinical consequences (1). BVDV also is considered to be one of the most significant potential contaminants in mammalian cell culture because of its ability to reproduce in cells without causing notable cytopathic effects. Serum collection, manufacturing, and treatment processes must be rigorous to minimize their risk of including such contaminating agents.
BVDV Pathogenesis
Transmission of BVDV occurs both through contact with infected cattle (horizontally) and from dam to calf (vertically) (2). These viruses can be transmitted through direct contact, through bodily secretions, and by contact with the environment or objects such as soil and clothing. BVDV can persist for at least two weeks in a cool and moist environment, especially when abundant organic material is present (3). Following viral contact with the mucosal lining of an animal’s mouth or nose, replication takes place in epithelial cells. BVDV infection causes a wide range of nonspecific clinical signs, including fertility loss, decreased milk production, pyrexia, diarrhea, and fetal infection. Although the clinical signs generally are mild, especially in cattle that have been previously exposed and/or vaccinated, a severe acute form of BVD occasionally does occur. Known as mucosal disease, that form is associated with high morbidity and mortality rates (4).
BVDV infection of a cow either just before she conceives or during the first 18 days of gestation leads to future delayed conception and an increased interval from calving to conception. Infection during days 29–41 can result in embryonic infection and/or subsequent embryonic death. BVDV can cross the placental barrier in cattle, so fetuses are exposed to the virus from their dams’ exposure (5).
If a fetus is exposed to the virus between 80 and 150 days of gestation — before developing an independent immune system — then that fetus can become “persistently infected” (PI) (6). PI animals remain infected throughout their lifetimes, with no ability to produce an immune response to the strain with which they have been infected. PI animals are the most important reservoirs of BVDV, continuously excreting it in large quantities over the course of their lives (7). In addition, PI dams always produce PI fetuses and calves (8). These animals often fail to thrive and end up smaller than their peers; however, occasionally they can appear normal.
Eradication: Vaccination and Culling
Several modified–live-viral (MLV) and inactivated BVDV vaccines are available on the veterinary market. Challenge studies indicate that they can prevent fetal infection under experimental conditions, but some experts question the efficacy of such vaccines under field conditions. A recent analysis of 45 studies in 40 published reports found that fetal infection in vaccinated animals posed roughly one-seventh the risk of unvaccinated controls regardless of the vaccine used (9). The risk was reduced further when considering only those studies involving a polyvalent vaccine or MLV vaccine.
Ultimately, the birth of PI calves into vaccinated herds suggests that both the type and timing of vaccinations can affect the efficacy of a vaccine when it is challenged by the transmitted viral load of PI cattle in the field.
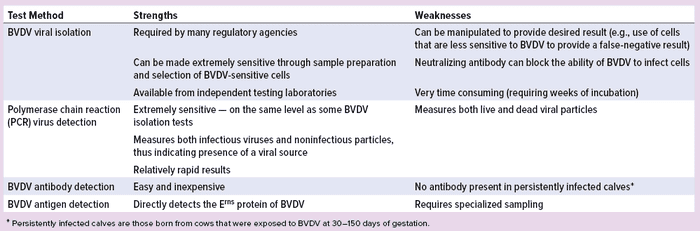
Table 1: Comparing test methods for bovine viral diarrhea virus (BVDV)
The mainstay of eradication for BVDV disease is identification and removal of PI animals. Further incidence of PI disease and occurrence can be prevented at least partially by vaccination and high levels of biosecurity supported by continued surveillance. Scandinavian countries lead the way in BVDV eradication, having implemented an eradication program nearly 20 years ago. It took them about 10 years to reach the final stages of eradication. To be effective, the program continues to require ongoing monitoring of all cattle herds within each country (10, 11). Currently, Scandinavia and Switzerland have completed successful eradication programs, and several other European countries have BVDV eradication programs ongoing (11–13).
Viral Classification
BVDV is an enveloped, single-stranded RNA virus, a member of the Pestivirus genus belonging to the family Flaviviridae. Three BVDV genotypes are recognized based on the nucleotide sequence of their 5′ untranslated region (UTR): BVDV-1, BVDV-2, and BVDV-3 (“HoBi”-like virus). BVDV-1 and BVDV-2 have been divided further into subgenotypes, with the former having a greater number than the latter. Finally, BVDV strains can be divided into distinct biotypes (cytopathic or noncytopathic) according to their effects on cell cultures (14):
BVDV1: Noncytopathic (ncp) viruses can induce persistent infection. They have intact NS2/3 proteins.
BVDV2: Formed through a mutation of ncp biotypes, cytopathic (cp) biotypes induce apoptosis in cultured cells. In a cp virus, either the NS2/3 protein is cleaved to NS2 and NS3 or a duplication of viral RNA contains an additional NS3 region.
A new strain of BVDV known as “Hobi”-like or atypical Pestivirus was isolated in 2004 from FBS originating in Brazil (15). Since then, this strain also has been isolated from cattle and water buffalo from South America (16). Currently, it is believed that the strain is prevalent across Brazil, but it also has been found in cattle elsewhere, notably in Thailand (17) and Italy (18). This new subtype of BVDV appears to be both cp and ncp, and evidence suggests that not all current testing paradigms (diagnostic and otherwise) can detect it (19).
Mitigation Strategies |
|---|
Virus-inactivating strategies in bioprocessing include gamma irradiation, UV-C irradiation, high-temperature–short-time (HTST) treatment of raw materials, and the use of virus-removal filters to reduce the risk of virus contamination in raw materials (1). Their implementation is in line with the expectations detailed in ICH Q5, which specifies that potential viral contamination should be controlled by the selection and testing of raw materials, including media components, for the absence of viral contaminants. But they are not all applicable to serum processing. To assess the capacity of downstream processes to inactivate/remove viruses, virus-spiking studies use downscale models of appropriate process steps (2–4). Common model viruses used in such studies include bovine viral diarrhea virus (BVDV), minute virus of mice (MVM), murine leukemia virus (MLV), porcine parvovirus (PPV), pseudorabies virus (PRV), and reovirus. BVDV is known to be more resistant to low-pH inactivation than both MLV and PRV (4). Complementary approaches for mitigating the risk of introducing infectious BVDV into a biomanufacturing process include gamma-irradiation pretreatment of serum by vendors and end users, treatment of media formulated with serum, and elimination of serum from culture media entirely (5). In some cases, the latter may not be possible — particularly for legacy products, cell lines that require serum for adequate productivity, and cell therapies that cannot be adapted to serum-free conditions. References1 Wisher M. Virus Risk Mitigation for Raw Materials: A European Perspective. BioProcess Int. 11(9) 2013: 12–15; https://bioprocessintl.com/upstream-processing/biochemicals-raw-materials/virus-risk-mitigation-for-raw-materials-347329. 2 Trapp A. Viral Safety in Intensified Monoclonal Antibody Processes. Am. Pharm. Rev. 20 July 2018; www.americanpharmaceuticalreview.com/Featured-Articles/352076-Viral-Safety-in-Intensified-Monoclonal-Antibody-Bioprocesses. 3 Ruppach H. Log10 Reduction Factors in Viral Clearance Studies. Bioprocessing J. 12(4) 2014: 24–30. 4 Challener C. Viral Clearance Challenges in Bioprocessing. BioPharm Int. 27(11) 2014: 42–44; www.processdevelopmentforum.com/articles/viral-clearance-challenges-in-bioprocessing. 5 Nims R, Plavsic M. The Pervasiveness of Bovine Viral Diarrhea Virus in Commercial Bovine Serum. Bioprocessing J. 11(4) 2012: 19–26; doi:10.12665/J114.Nims. |
BVDV in FBS
BVDV occurs in herds around the world with an overall consistent incidence globally. Infection levels are influenced by cattle density and (to a lesser degree) season. Prevalence of PI cattle range from ≤0.3% (20) to 2% (21) in herds worldwide. Because manufactured lots of serum are pooled in ~2,000-L batches, one liter of FBS will end up representing several animals.
The incidence of BVDV in serum follows basic principles of statistical binomial probability:
If a pool of 1,000 animals is used at 1% incidence, then the pool will be positive 100% of the time.
If a pool of 100 animals is used at 1% incidence, then the pool will be positive 60% of the time.
If a pool of 20 animals is used at 1% incidence, then the pool will be positive 20% of the time.
Hence, it is possible to reduce BVDV burden in FBS by reducing the number of animals represented by each pool. Small pools can be pretested for BVDV and selected to reduce the risk of contamination further. Although this approach is effective, it is also costly.
Postmanufacturing Treatment
A number of methodologies have been used in attempts to reduce the viral burden of bovine serum. BVDV is highly susceptible to gamma irradiation, the method of choice for reducing the postmanufacturing viral load. A dose of 30 kGy has been shown to reduce it by 106 (22).
Gamma irradiation targets viral genomes and can cause strand breaks and other types of damage to nucleic acids. So when gamma irradiation has been implemented, it is essential not to use nucleic-acid–based analytical tools such as polymerase chain reaction (PCR) for determining virus titer. Although viruses will be inactivated completely (rendered noninfectious), such testing methods still will detect fragments of the viral nucleic acids that remain, making it impossible to interpret study results. Thus, cell-based infectivity methods must be chosen for virus titration when gamma irradiation has been used.
Virus reduction titers are analyzed using assays such as TCID50 (the concentration at which 50% of cells are infected when cell cultures are inoculated with a diluted solution of virus-containing fluid), plaque-forming units (PFUs, the number of virus particles capable of forming plaques in cell cultures), and fluorescent-focus assays (FFAs). Such methods should be validated at least as for specificity and sensitivity (limit of detection, LoD), and their limitations should be well understood.
A number of tests can be used for BVDV, with the most common being virus isolation, detection by PCR, antibody detection, and antigen capture (23, 24). Table 1 compares their strengths and weaknesses.
Virus Isolation: Virus isolation has been the “gold standard” in BVDV detection for some time. BVDV-susceptible cells are incubated in the presence of a serum sample and then subsequently analyzed for the presence of BVDV.
PCR Detection: RNA is extracted from a given serum sample, then converted to DNA and replicated up to 50 times. This assay also can be used to quantify viral particles.
Antibody Detection: The presence of BVDV immunity is detected with enzyme-linked immunosorbent assays (ELISAs). They do not diagnose active infection, but rather, they detect the presence of antibodies produced by animals in response to viral infection and/or vaccination.
Antigen Detection or Antigen Capture: This method detects BVDV Erns antigen directly and serves as the basis for PI detection.
Regulations
Determining the absence or presence of BVDV in serum is regulated by government and pharmaceutical agencies. Because BVDV infection is a worldwide disease with little difference in incidence rates among countries around the globe, some experts consider restrictions on importation of animal serum based on its presence in importing countries to be unnecessary for protecting animal health. However, many countries require that serum be tested for BVDV before it can be imported.
Most requirements for BVDV testing in the pharmaceutical arena are highly detailed and designed for maximum sensitivity. It is common practice to use BVDV viral-isolation assays to protect cell lines in this sector; however, it must be noted that no standardized reagents have been prescribed for such testing (e.g., susceptible/permissive cell lines, antibodies for immunofluorescent detection, and so on), nor do regulatory agencies agree on exactly what testing should be conducted.
Currently, multiple cell lines are in use, as we determined through a literature review of research published since 1963. These cell lines have been shown to be both permissive and susceptible to BVDV infection and subsequent viral replication: bovine embryo kidney (BEK) cells, primary bovine fetal kidney (BFK) cells, primary and secondary bovine embryo testicle (BET) cells, fetal bovine bone-marrow cells, Madin-Darby bovine kidney (MDBK) cells, bovine turbinate cells, and primary fetal bovine lung cells. No known bovine cell line is nonpermissive or nonsusceptible to BVDV. And although it would appear that the vast majority of (if not all) bovine cell lines will support amplification of BVDV virus, the extent to which they can do so varies.
We highly recommend that the LoD for each BVDV type (1–3) be known for each cell line used. That way, FBS end users will know the likelihood of detecting for BVDV under the testing conditions they use. Finally, the presence of anti-BVDV neutralizing antibodies can block infection in cell cultures, leading to false-negative results. Care and proper vetting should be part of the selection process if a company chooses to outsource this testing to a contract-service laboratory.
BVDV-Free Serum
The ideal for BVDV testing is virus isolation, but the assay has no established standard of sensitivity. Serum screened in small batches can be pooled to create a dilution effect that allows it to pass testing as “BVDV free.” That would be described more accurately as “BVDV free at the sensitivity LoD of the assay used.” Although raw serum also can be gamma irradiated or treated with BVDV antibody to ensure a negative result in a viral isolation test, gamma irradiation of raw serum is not a preferred method. Note that even correctly performed gamma irradiation of finished product might not produce true “BVDV-free” serum, depending on the initial viral load. True BVDV-negative serum must test negative by both PCR and virus-isolation methods. To eliminate factors that complicate interpretation, several tests should be used in tandem to determine the true status of serum and the means (if any) by which it has been rendered “BVDV free.”
References
1 Fray MD, Paton DJ, Alenius S. The Effects of Bovine Viral Diarrhoea Virus on Cattle Reproduction in Relation to Disease Control. Anim. Reprod. Sci. 60–61, July 2000: 615–627; doi:10.1016/s0378-4320(00)00082-8.
2 Houe H. Epidemiology of Bovine Viral Diarrhea Virus. Vet. Clin. N. Am. Food Anim. Pract. 11(3) 1995: 521–547; doi:10.1016/s0749-0720(15)30465-5.
3 Bøtner A, Belsham GJ. Virus Survival in Slurry: Analysis of the Stability of Foot-and-Mouth Disease, Classical Swine Fever, Bovine Viral Diarrhoea, and Swine Influenza Viruses. Vet. Micro. 157(1–2) 2012: 41–49; doi:10.1016/j.vetmic.2011.12.010.
4 Meyling A, Houe H, Jensen AM. Epidemiology of Bovine Virus Diarrhoea Virus. Rev. Sci. Tech. 9(1) 1990: 75–93; doi:10.20506/rst.9.1.489.
5 Moennig V, Liess B. Pathogenesis of Intrauterine Infections with Bovine Viral Diarrhea Virus. Vet. Clin. North Am. Food Anim. Pract. 11(3) 1995: 477–487; doi:10.1016/s0749-0720(15)30462-x.
6 McClurkin AW, et al. Production of Cattle Immunotolerant to Bovine Viral Diarrhea Virus. Can. J. Comp. Med. 48, April 1984: 156–161.
7 Innocent G, et al. A Computer Simulation of the Transmission Dynamics and the Effects of Duration of Immunity and Survival of Persistently Infected Animals on the Spread of Bovine Viral Diarrhoea Virus in Dairy Cattle. Epidemiol. Infect. 119(1) 1997: 91–100; doi:10.1017/s0950268897007723.
8 Binkhorst GJ, et al. Neurological Disorders, Virus Persistence, and Hypomyelination in Calves Due to Intra-Uterine Infections with Bovine Virus Diarrhoea Virus. Vet. Q. 5(4) 1983: 145–155.
9 Newcomer BW, Chamorro MF, Walz PH. Vaccination of Cattle Against Bovine Viral Diarrhea Virus. Vet. Micro. 206, July 2017: 78–83; doi:10.1016/j.vetmic.2017.04.003.
10 Hult L, Lindberg A. Experiences from BVDV Control in Sweden. Prev. Vet. Med. 72(1–2) 2005: 143–148; doi:10.1016/j.prevetmed.2005.04.005.
11 Moennig V, Houe H, Lindberg A. BVD Control in Europe: Current Status and Perspectives. Anim. Health Res. Rev. 6(1) June 2005: 63–74; doi:10.1079/ahr2005102.
12 Moennig V, Greiser-Wilke I. [Perspectives on BVD Eradication in Germany]. Berl. Münch. Tierärztl. Wochenschr. 116(5–6) 2003: 222–226.
13 Presi P, Heim D. BVD Eradication in Switzerland: A New Approach. Vet. Micro. 142(1–2) 2010: 137–142; doi:10.1016/j.vetmic.2009.09.054.
14 Baker JC. Bovine Viral Diarrhea Virus: A Review. J. Am. Vet. Med. Assoc. 190(11) 1987: 1449–1458.
15 Schirrmeier H, et al. Genetic and Antigenic Characterization of an Atypical Pestivirus Isolate, a Putative Member of a Novel Pestivirus Species. J. Gen. Virol. 85(Pt 12) December 2004: 3647–3652; doi:10.1099/vir.0.80238-0.
16 Stalder HP, et al. Genetic Heterogeneity of Pestiviruses of Ruminants in Switzerland. Prev. Vet. Med. 72(1–2) 2005; 37–41; doi:10.1016/j.prevetmed.2005.01.020.
17 Ståhl K, et al. Natural Infection of Cattle with an Atypical “Hobi”-Like Pestivirus: Implications for BVD Control and for the Safety of Biological Products. Vet. Res. 38, May–June 2007: 517–523; doi:10.1051/vetres:2007012.
18 Decaro N, et al. Hobi-Like Pestiviruses in Aborted Bovine Fetuses. J. Clin. Micro. 50(2) 2012: 509–512; doi:10.1128/JCM.05887-11.
19 Bauermann F, Flores E, Ridpath J. Antigenic Relationships Between Bovine Viral Diarrhea Virus 1 and 2 and Hobi Virus: Possible Impacts on Diagnosis and Control.
J. Vet. Diagn. Invest. 24(2) 2012: 253–261; doi:10.1177/1040638711435144.
20 Van Campen H. Epidemiology and Control of BVD in the U.S. Vet. Micro.
142(1–2) 2010: 94–98; doi:10.1016/
j.vetmic.2009.09.049.
21 Benfeldt S, et al. [Seroprevalence in Four Different Counties in Lower Saxony and the Relevance for the Control Strategy]. Tierärztl. Umsch. 59, 2004: 499–507.
22 Plavsic M, et al. Gamma Irradiation of Animal Serum: Validation of Efficacy for Pathogen Reduction and Assessment of Impacts on Serum Performance. Bioprocessing J. 15(2) 2016: 12–21; doi:10.12665/J152.
23 Grooms, et al. Diagnosis of BVDV: A Key Component to a Comprehensive BVDV Control Program. Michigan State University College of Veterinary Medicine: East Lansing, MI, 2005; https://www.ars.usda.gov/ARSUserFiles/50302000/BVD2005/Produce2_Grooms_Hout.pdf.
24 Lindberg ALE. Bovine Viral Diarrhoea Virus Infections and Its Control: A Review. Vet. Q. 25(1) 2003: 1–16; doi:10.1080/01652176.2003.9695140.
Steven Doelger is director of operations at the International Serum Industry Association (ISIA); Lisa Orfe is microbiology laboratory manager at VMRD Inc.; Michelle Cheever is director of operations at Atlas Biologicals; Randy Fitzgerald is president of RCC Consulting LLC; and Greg Hanson is regulatory affairs manager for cell culture at HyClone Laboratories LLC. Corresponding author Rosemary J. Versteegen is chief executive officer of ISIA, PO Box 196, McHenry, MD 21541; 1-301-387-4967; [email protected]; www.serumindustry.org.
You May Also Like






