I write this the day after US President Joe Biden’s address reporting his administration’s progress on COVID-19. Vaccines and their distribution and delivery are priorities, of course, and the companies working on them over the past year deservedly have received a great deal of attention. But our pandemic response rests on three other important pillars as well: prevention of exposure through masking and social distancing (as the President reminded us), testing and tracing with diagnostics and data, and treatment of those patients who do become infected. And even though Biden did not mention the latter, the biopharmaceutical industry has a lot to offer on that front after a year of working on treatment approaches behind the scenes.
This issue features one treatment under development in India: hyper-immunoglobulin G antibodies purified from the plasma of convalescent patients. Although convalescent plasma has been used as a relatively inexpensive treatment for other infectious diseases, it has not been a su...
On 21 July 2020, the US Attorney’s Office in Spokane, WA, unveiled a sweeping indictment accusing a pair of Chinese hackers of conspiring to steal trade secrets from a number of American pharmaceutical companies and research institutions, including biotechnology companies working on potential COVID-19 vaccines (
1
). Although the hackers apparently did not succeed in their attempt to obtain vaccine data, their years-long conspiracy involved other attacks on the pharmaceutical industry — and netted them troves of sensitive commercial information. Among the stolen data were design specifications and test results, toxicity information, and dosing research about a California company’s therapy for a chronic condition. The hackers also pilfered source code used in a Massachusetts company’s medical devices as well as algorithms essential to their operation.
Although the Spokane case still is in its preliminary investigative phase, that indictment follows a wave of similar cases, both civil and criminal, involvin...
The Center for Biopharmaceutical Education and Training (CBET) at the Albany College of Pharmacy and Health Sciences is centrally located at the NanoTech Complex in Albany, NY.
(Photo Credit:
NY Creates
)
Beyond the human suffering and economic damage caused by COVID-19, one of the most powerful results of the pandemic has been to focus global attention on drug and vaccine development for infectious diseases. Massive investments by governments, institutions, and biopharmaceutical companies have accelerated development of novel therapeutics, including messenger RNA (mRNA) and viral-vector vaccines, that are poised to become transformational platform technologies for biopharmaceutical manufacturing (
1
). Furthermore, the well-established technology of monoclonal antibodies (MAbs) has lived up to its promise to deliver treatments and potentially preventive therapeutics for the SARS-CoV-2 virus (
2
).
Such dynamics will intensify what was already a challenging constraint facing the biopharmaceutical industr...
This two-day CASSS CMC Strategy Forum explored many technical, practical, and regulatory facets of biological drug-product (DP) analytics, process validation, and comparability.
Part 1
of this report summarized the discussions on drug-product analytics and comparability in BPI’s March 2021 issue (
1
). Here we report on day two presentations and discussions on validation, legacy products, and lifecycle management.
Session Three: Drug-Product Validation
The morning session focused on principles of process validation with examples of challenges specific to drug products.
New Risk-Based Process Performance-Qualification Strategies:
Patrick Donahue of Janssen began by reviewing the US Food and Drug Administration’s (FDA’s) guidance on process validation (
2, 3
). It introduced the concept of a control strategy driven by relative risk and mitigation that is commensurate to the type and level of risk.
Criticality of an item (e.g., parameter, process, or equipment) is the guidepost for monitoring and control:...
Biopharmaceuticals produced from mammalian cell cultures are susceptible to viral contamination. That risk is mitigated by applying complementary approaches. Those include extensive testing of cell banks, selecting low-risk raw materials, testing cultivations for viruses, and documenting the capacity of a purification process to inactivate and remove viral contaminants. The latter commonly is referred to as
viral clearance
and usually expressed as a log reduction value (LRV).
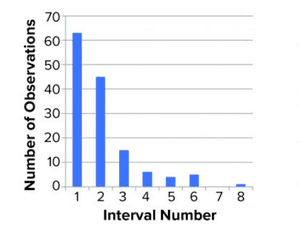
Figure 1: Distribution of titer (left) and log(titer) (right) values; we performed 139 determinations of titer of a stock positive-control material. The range (lowest to highest value) was divided into eight equally sized intervals (
1–8
), and the number of data points within each interval was counted and shown in a histogram. For a normal distribution, a symmetrical distribution around a mean value is expected.
Novo Nordisk has performed several viral clearance studies for different processes and process steps. The availability o...
The novel severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) emerged as a major pandemic coronavirus disease in 2019 (COVID-19) and since then has killed many people and paralyzed the global economy (
1,
2
). With specific antiviral therapeutic agents or antibodies yet to be approved, other antivirals and novel vaccine strategies have been essential to containing the virus and disease transmission.
Passive antibody therapy can be used to limit the scope of epidemics by providing patients with antibodies that recognize epitopic regions of virus particles to reduce their replication and consequently lessen the severity of viral disease. Antibodies for passive immunotherapy can be manufactured through recombinant DNA technology or isolated from the blood of recovered patients. The conventional strategy using convalescent plasma (CP) isolated from treated patients has improved survival rates in several viral infection events involving Machupo virus (Bolivian hemorrhagic fever) (
3
), Junín virus ...
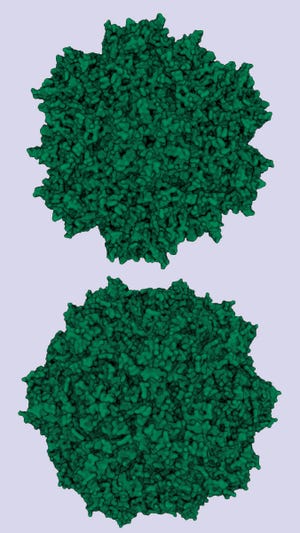
Similarity in structures of adenoassociated virus serotype 8 (top) and minute virus of mice (bottom).
Photo credit: Protein Data Bank
(
https://www.rcsb.org
)
Over the past decade, adenoassociated virus (AAV) vectors have become established as leading gene-delivery vehicles. In 2017, the pipeline for gene therapies included 351 drugs in clinical trials and 316 in preclinical development (
1–4
). As those candidates advance, significant efforts are being made in process development and manufacturing for viral vectors, with the overall goal of reducing process impurities while maintaining the highest possible process yield.
To address that goal, industry suppliers have developed innovative AAV-specific separation technologies. Thermo Fisher Scientific’s POROS CaptureSelect AAVX affinity resin provides a capture method for a number of natural and synthetic AAV serotypes irrespective of the expression system used to produce them. By leveraging a proprietary recombinant camelid antibody technology immobilized...
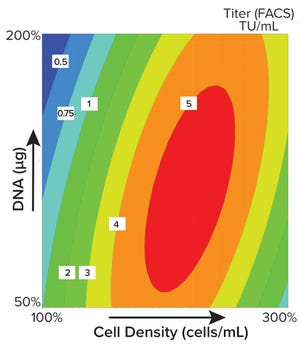
Figure 1: Contour plot obtained using MODDE software shows the optimal seeding density for viral vector production. The quantity of plasmid DNA (µg) is plotted against cell density (cells/mL) with increasing infectious titer over that of the initial process as the response, in this case, increasing from 0.5 (blue) to 5 (red); FACS = fluorescence-activated cell sorting, TU = transduction units.
With recent developments and successes in cell and gene therapy, the biopharmaceutical industry is facing increased demand for safe and efficient delivery systems (
1
). Viral vectors, including adenoviruses (AV), adenoassociated viruses (AAV), and lentiviruses (LV), are among the most common delivery agents because they infect mammalian cells efficiently. Suspension cultures have become a popular choice for robust and scalable viral manufacturing systems. Using stable cell lines that integrate all or part of the viral production elements adds further benefits by improving consistency and reducing manufacturing cost...
Interest in industrial-scale production of messenger RNA (mRNA) has surged amid rapid development of mRNA-based vaccines against SARS-CoV-2. During an
18 February 2021 Ask the Expert presentation,
Aleš Štrancar
(chief executive officer of BIA Separations, a Sartorius company) reminded attendees that no platform approach yet exists for mRNA production and that much remains to be learned about manufacturing such products at commercial scales. He described current production challenges and shared BIA’s efforts to devise flexible mRNA purification tools.
Štrancar’s Presentation
Understanding Reagents:
Štrancar emphasized the need for impurity control during mRNA manufacturing. The process usually entails isolation of plasmid DNA (pDNA), plasmid linearization, mRNA production by in vitro transcription (IVT), and finally, mRNA purification. Poor understanding of interactions among reagents can diminish mRNA yield and purity. For instance, quality of raw materials varies across lots. Key enzymes can aggregate...
Process development teams have relied for decades on benchtop bioreactors to perform mammalian-cell–culture experiments; however, doing so requires significant resources and staffing. During a 24 February 2021 presentation,
Deborah Pascoe,
PhD, vice president of operations at Culture Biosciences (Culture), explained how to leverage her company’s high-throughput, cloud-connected bioreactors to execute Chinese hamster ovary (CHO) cell cultures with high reproducibility and scalability.
Pascoe’s Presentation
Pascoe described her company as an extension of a customer’s laboratory. After technology transfer, Culture and a client enter into an ongoing guaranteed-capacity agreement specifying a certain number of bioreactor runs per month. Clients submit experimental designs; then Culture executes them in its 250-mL cloud-connected bioreactors. Culture’s Console analytics platform presents the resulting data in real time, and process samples are sent to clients monthly.
Such a business model combines the advant...
Having brought its first vaccine candidates from concept to clinical development in less than three months, BioNTech is making global waves as the pioneer behind Pfizer’s COVID-19 vaccine. Because of such success stories, more eyes than ever are looking at opportunities raised by the biotechnology industry. As talented people around the world from academia, clinical practice, and other industries consider careers in this sector, they should examine the characteristics necessary to become successful biotechnology entrepreneurs.
Biotechnology fuses science and business. It is a major global economic driver that, according to Statista, generated US$140 billion in revenue in 2016 (
1
). Biotech companies employ millions of people around the world, and demand for skilled professionals continues to rise. However, the sector creates different challenges from those facing other industries. It also works within long, complex timelines for product development and clinical trials.
So what is most important to the li...
Tosoh Bioscience’s SkillPak 1-mL and 5-mL prepacked columns
Some of the latest promising biopharmaceutical drug substances are antibody fragments. Antibody fragments are either separate functional subunits of antibodies or recombinant molecules, which, just like antibodies, are composed of immunoglobulin domains. These drugs offer several therapeutic advantages over conventional monoclonal antibodies.
Upstream processing for antibody fragments is easier than it is for standard antibodies. Recombinant-based antibody fragments can be modified to meet specific needs of affinity, avidity, valence, and action mode. They also can be produced in prokaryotic cells (such as
Escherichia coli
), a cell line more economic than mammalian expression systems. Downstream processing, however, can be challenging. As antibody fragments do not have an Fc-region, chromatography experts cannot use protein A affinity and must select protein L as a ligand for fragment capture. Protein L is a surface protein of
Peptostreptococc...