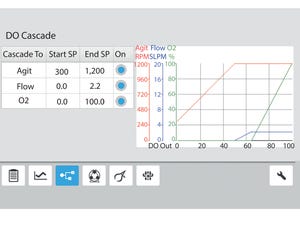
Learning to use a bioprocess controller is a complex endeavor. Even if someone has previous bioprocess experience, moving to a new software platform can entail much learning and reduce process efficiency. With the auto-culture modes of the Eppendorf BioFlo 120 bioprocess control station, a user can select with a push of a button either a predefined
Escherichia coli
batch fermentation protocol or a Chinese hamster ovary (CHO) batch cell culture process.
Proof of Concept:
E. coli
Auto-Culture Fermentation
The auto-culture modes are populated with setpoints tested by the Eppendorf applications development team. A user needs only to select the vessel size and type and make standard preparations (e.g., sensor and pump calibration, vessel preparation). Once ready, the user simply presses the “play” button. To demonstrate the ease of use of the auto-culture mode, we performed an
E. coli
batch fermentation using a strain that produces green fluorescent protein (GFP). The setpoints for each control loop were...
There are no definitive requirements on how to perform extractables and leachables (E&L) studies. However, the US Pharmacopeial Convention recently released two chapters to provide guidance on performing E&L studies. USP chapters <1663> and <1664> discuss options and considerations in performing an extractables study and a leachables study, respectively. There are also various workgroups that have published guidance documents to assist industry in designing and performing these studies. The Product Quality Research Institute (PQRI), the Bio-Process Systems Alliance (BPSA), and the Bio-Phorum Operations Group (BPOG) all have released publications defining best-practices approaches to perform the testing. The PQRI guidance document focuses primarily on performing studies on orally inhaled and nasal drug products. Both BPSA and BPOG focus on the evaluation of single-use technology. Although the general approaches to generating an extractables profile of a contact material are similar, the details in performi...
Original developers of biosolutions and products, especially those facing the debut of biosimilars in core markets, have an urgent imperative to reduce manufacturing costs through increased productivity and yields. In turn, this drives a wide range of business decisions, including capital investment, process choices and design, and equipment selection. To this end, biodevelopers are adopting more sophisticated processes (such as perfusion) to address low-titer cell lines and reduce raw material costs. They’re also seeking more sophisticated and flexible R&D and process development capabilities in several ways by using equipment to enable simultaneous development of multiple products, automate rapid experimental design and implementation, optimize processes, and gain better analytical insights — especially for process analytical technology (PAT) and regulatory compliance.
Introducing AlphMab, a Fully Equipped Biodeveloper and Producer
AlphaMab Co. Ltd. is a fast-growing biodeveloper and producer with those...
Steve Bagshaw, chief executive officer at FUJIFILM Diosynth Biotechnologies, offers some insight into the ever changing CDMO landscape and the future of medicine. Mr. Bagshaw is an industry thought leader and an advocate of the Biotechnology Industry.
Q: Fujifilm Diosynth Biotechnologies has been expanding and making big announcements in the past year. Can you talk about how this growth is shaping your long-term vision as a contract development and manufacturing organization (CDMO)?
We are seeing the benefits of long-term investment. In 2013, we launched our Apollo mammalian expression technology, which was the end result of an in-house innovation program. The Apollo technology has been incredibly successful. As a leading CDMO, our process development is one of our key differentiators. We want to maintain and expand our technical leadership long term by investing with the addition of new state of the art laboratories.
Login to read the full PDF of this article.
Sponsored Content
There is now a larger selection of the world’s first controllable single-use diaphragm valve: GEMÜ SUMONDO.
GEMÜ, the leading manufacturer of valve designs for the pharmaceutical industry, has established the first controllable single-use diaphragm valve on the market: the GEMÜ SUMONDO. In addition to a pneumatically operated version, the product range now includes a version with a hand wheel for manual operation. And because of increased customer demand globally, the product range has been expanded in the area of associated valve bodies. With a third diaphragm size now introduced, another high-performance option has been added to the product range: the largest valve of its type to date, up to one inch in process connection size. That means that applications that require a higher medium flow and precise controllability now have a solution.
Login to read the full PDF of the application note.
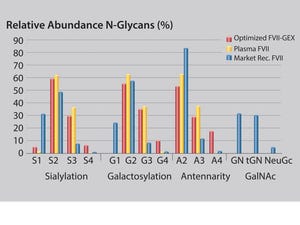
GlycoExpress® (GEX®) technology is a well-established human expression platform for the screening and production of biotherapeutics. By contrast with other human cell lines (e.g., HEK293 and Per.C6) GlycoExpress® expression platform consists of a toolbox of glycoengineered cell lines optimized for producing biotherapeutics with desired glycan structures to improve their clinical performance.
Glycotope’s GEX® technology provides an established expression platform proven for different biopharmaceuticals:
Login to read the full PDF of the application note.
Single-use powder containment is vital in today’s safety-focused manufacturing environment. Preventing product from cross-contamination and reducing airborne particulates is a major concern. Understanding how modern bag designs can increase safety and speed is essential for the industry. We looked at the ease of filling along with dispensing times and product loss. ILC Dover’s EZ BioPac® system shows a 71% decrease in filling times when measured against an industry-standard 2D bag. Because of the nonstatic film, we saw an almost 20% reduction in powder discharge times. In addition, it demonstrates a 33.3% improvement in powder recovery, showing reduced waste. Choosing the proper containment system can have a significant impact on plant productivity and profitability.
Issues of Powder Handling
Historically, powdered media and buffer ingredients were transferred from stock containers using open scoops, weighed and mixed in buckets, and then carried in and dumped from those buckets directly into production v...
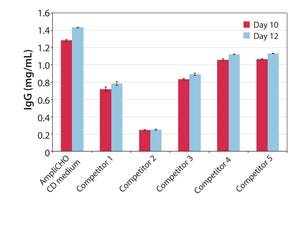
For more than 75 years, Kerry has earned its reputation for reliability and excellence in serving the biotechnology, pharmaceutical, and nutrition markets. We deliver innovative solutions to assist customers increase cell proliferation, extend cell viability, and increase target protein production in biotechnological production systems.
Every day we expand our capabilities to meet the changing needs of the biotech market. Kerry’s products have evolved with market trends in cell culture over the past fifty years to fulfill our customers’ requirements. Those products have grown to encompass modern plant-based hydrolysates, yeast extracts, recombinant proteins, complex supplements systems, and chemically defined media. We have the vast global resources and technical platform to deliver consistent, high-quality products backed by unparalleled service, technical support, and formulation customization capabilities.
Login to read the full PDF of the application note.
MaxCyte’s delivery platform is a universal, high-performance transfection technology that significantly reduces risk and shortens biotherapeutic development timelines by enabling researchers to
Gram-Scale Production of Quality Antibodies:
MaxCyte’s Flow Electroporation™ technology is a fully scalable, high efficiency means of transiently producing milli- to multigram quantities of antibodies in the host cell line-of-choice without the need for specialized reagents, vectors, or engineered cell lines. CHO cell transfection efficiencies and cell viabilities of >95% enable rapid, high-titer expression of antibodies (Figure 1) and antibody-like molecules. The high level of antibody quality in combination with the seamless scalability and regulatory pathway of MaxCyte’s delivery platform uniquely fulfill the needs of R&D activities through CGMP pilots and toxicology studies.
What factors impact peptone variability? Variability can be linked to at least two critical elements: the biological raw materials and the production site. Raw materials can vary by species of animal or plant sourced, type of components (specific animal tissues as opposed to standardized materials), and geographical origins. Feeding practices also can cause variability. Within a single manufacturer, interbatch variability can be linked to the condition and age of a production site, its level of automation and sophistication, optimized production protocols, the types of products being processed, and the amount of human intervention.
Tangible Demonstrations of Consistency
Appropriate demonstrations of consistency can be found in batch reviews of actual productions. Microbial models can prove challenging in light of variables in media composition and the particularity of the microorganisms themselves. Total nitrogen is especially pertinent because it represents the main objective of the peptone: its contribu...
Sponsored Content
Bovine serum albumin (BSA) is a critical component of many biotechnology and biochemistry systems. BSA is used in a variety of applications, including diagnostics, veterinary medical products, vaccine manufacturing, mammalian cell growth for biopharmaceutical production, and topical and ex vivo medical device applications. The impact of BSA on the performance of these systems is often overlooked and is identified as a possible root cause of variability only after extensive testing and elimination of other system components.
There are many different functions of BSA within the spectrum of biopharmaceutical applications. Diagnostic tests for both animal and human health industries use BSA as a protein carrier to stabilize low-abundance high-value proteins (e.g., monoclonal antibodies). BSA is used as an assay standard given the reproducible properties of a pure protein fraction and as a blocking agent to prevent nonspecific interactions with the functional components of the assay. BSA also is used as an ine...
Pichia pastoris (Komagataella phaffii)
is recognized as a highly competitive expression system for fast and economic production of recombinant proteins. Pichia offers many advantages. It is an established (FDA and EMA approved), safe (generally recognized as safe, GRAS), and highly competitive expression host with strong and effective secretory capacities often resulting in double-digit grams-per-liter product levels in culture supernatant. It combines advantageous properties of prokaryotes and mammalian cells. On one hand, Pichia cultures grow fast and can reach high cell densities on inexpensive and chemically defined media. On the other hand, the organism has a subcellular protein processing machinery, which is required for posttranslational modification and active secretion of proteins. Simplicity of genetic manipulation and high productivity paired with the capability to secrete recombinant proteins efficiently further underline the capabilities of
P. pastoris
for both R&D and commercial productio...
Standardizing equipment in biopharmaceutical production streamlines operations, saving end users time and money. An area ripe for standardization is the use of genderless connections, those in which the connector halves are identical in design. Historically, single-use connections have involved male and female halves, but using two different parts can create several inefficiencies and complications, including
Login to read the full PDF of the application note.
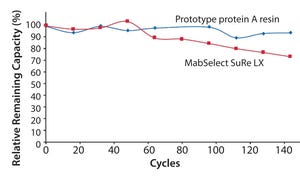
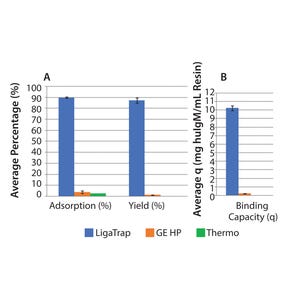
It’s been over 30 years since the identification and commercialization of protein A. Since its inception, protein A has served as the pinnacle purification platform for the immunoglobulin G (IgG) subclass of antibodies, both monoclonal and polyclonal forms. The introduction of protein A paved the way for researchers and large pharmaceutical manufacturers to develop and commercialize IgG-based therapeutics at both small and large scales, provided by its relative ease of use and well-characterized chromatographic attributes. Unfortunately, the gold standard affinity ligand, protein A, does not bind to all immunoglobulin subclasses, including immunoglobulin M (IgM), thereby forcing those working with IgM to find alternative purification strategies and leaving IgM to hitchhike on that “yellow brick road” that protein A offers so readily for IgG.
Moreover, little to no advancements have been made in developing a robust IgM purification platform to date. Therefore, the emergence of IgM-based therapeutics has be...
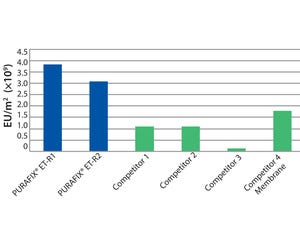
Germany-based em-tec GmbH has been developing and manufacturing products and components for the medical and bioprocessing industry for decades. With many years of experience, em-tec has become known as a strong partner for consulting, development, and production focusing on noninvasive flow measurement systems using the ultrasound transit time principle. The long-established and medically certified bidirectional clamp-on flow measurement system for liquids has been adapted for the special needs of the good manufacturing practice (GMP)–oriented bioprocessing industry.
The Flow Measurement System for Bioprocessing and Pharmaceutical Processes
The BioProTT™ flow measurement system consists of a disinfectable Clamp-On transducer and a FlowTrack flowmeter with the measuring electronics. Flexible tubing can be easily inserted into the Clamp-On transducer. After the lid is closed, the measurement can start without splicing the tubing lines. There is never contact between the sensor and the measured medium. The ...
Endotoxins are degradation products from dying gram-negative bacteria and complex aggregates of acidic lipopolysaccharides (LPS). Each is composed of lipophilic lipids and hydrophilic polysaccharides. In humans, endotoxins can cause immune responses such as fever (pyrogenic threshold is approximately 0.1 ng/kg body weight). Unlike bacteria themselves, endotoxins are extremely heat and pH stable and therefore withstand sterilization methods.
During protein purification, the reduction of endotoxins is one of the most important and difficult steps. It often includes complex purification strategies (e.g., chromatography steps) with more or less satisfactory results.
Login to read the full PDF of the application note.
Bioburden control is an area of serious concern for both manufacturers of biologicals and suppliers to the industry. This white paper considers some of the risks related to downstream processing and presents recent developments by suppliers that help manufacturers mitigate these risks. Topics covered include improvements in raw material quality, equipment design, chromatography resin properties, and ways of working. Challenges specifically related to the sanitization of protein A chromatography resins also are discussed.
Bioburden Control in Biopharmaceutical Production
The risk of microbial contamination is inherent in most production scenarios for biologicals. Conditions in the upstream process, with controlled temperature and a nutrient-rich medium, support not only growth of the target cells, but also of other biological organisms.
Downstream operations also have their share of challenges. Open containers can be used to transfer product or handle buffers. Raw materials and processing aids that have co...
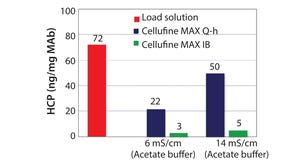
Cellulose is well-known as a natural raw material that has mechanical strength, lower nonspecific adsorption, and good biocompatibility. Additionally, cellulose particles have unique pore-size characteristics appropriate for the chromatography of biopharmaceuticals. Mixed-mode chromatography resins are well known to have unique selectivity differences from traditional IEX or HIC resins. Cellufine™ MAX IB, a novel cellulose-based mixed-mode resin, has polyallyl amine partially modified with butyl groups ligand (Figure 1). The resin is used in flow-through mode after a protein A step in monoclonal antibody (MAb) purification. Superior performance of impurities removal from MAbs with this resin can provide two-step MAb purification.
Host-Cell Protein (HCP) Removal with Cellufine™ MAX IB and Polymer-Modified Q AEX After ProA Step:
Cellufine™ MAX IB resin exhibits a salt-tolerance property derived from polyamine ligand. In general, high ionic strength decreases adsorption performance of ion-exchange (IEX) res...
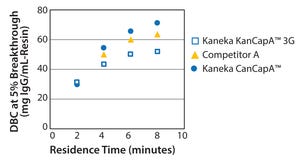
Recent improvements in monoclonal antibody (MAb) upstream process technologies have led to increased product titers (from 5 to 10 g/L) and a corresponding change in impurity levels. To yield highly pure MAb drugs from such high-titer feedstocks, new, robust, and cost-effective purification processes need to be developed to follow this upward trend.
KANEKA has developed KANEKA KanCapA™ 3G, an innovative protein A resin that demonstrates higher binding capacities and allows milder elution conditions than well-known high-binding capacity agarose based resin.
Login to read the full PDF of the application note.
LEWA EcoPrime® Twin LPLC is an easy-to-use, GMP-ready, multicolumn chromatography system offering analytical performance for continuous chromatography from process development to large-scale biopharmaceutical production. The EcoPrime Twin platform spans a wide range of flow and column sizes and has several options to incorporate multiple functionality on a single unit. The EcoPrime Twin system is built on ChromaCon’s Contichrom patented approach to twin-column purification. Because of the two-column configuration, the EcoPrime Twin system is a simple design that facilitates continuous chromatography implementation, reduces risk of costly downtime, and minimizes operating expenses. It is well suited for capture steps using protein A or ion-exchange (IEX) separations for monoclonal antibody, recombinant, or plasma protein purification processes.
One System, Multifunction
This advanced system can be configured to operate in multiple modes: batch and continuous chromatography with enhanced buffer in line dilu...
With the demand of higher production and reducing cost in biological manufacturing, continuous bioprocessing is replacing the traditional batch-mode manufacturing. Many biopharmaceutical companies are seeing the benefits of continuous processing. Novasep has enabled fully integrated continuous bioprocessing — from perfusion bioreactor to formulation.
Objective
This application note highlights a study conducted with Novasep’s BioSC Lab® technology for a fully continuous monoclonal antibody (MAb) production. BioSC Lab® is a sequential multicolumn chromatography (SMCC) system integrated as the capture step using protein A resin. BioSC® technology enables processes to run at higher linear flow rates while effectively using almost 100% of the capacity of a resin. The end result is a significantly improved productivity (gram of protein per liter of stationary phase per day), as well as significant reduction in buffer consumption (liters of buffer per gram of protein). Because of the increased productivity, the ...
Since the introduction of single-use technologies (SUTs) more than 20 years ago, their benefits have been well recognized, and their adoption has been swift.
The ability to customize a solution for every operation, every scale, and every product is an attractive proposition when compared with the fixed, stainless-steel facilities SUTs have replaced. But now customization has gone too far. Custom assemblies are being used when there is no need or benefit, and excessive customization is becoming a key challenge of implementing SUTs in biomanufacturing.
Consequences of Excessive Customization
Too much customization creates complexity. One challenge is the need to stock and control many low-volume items, including managing and writing off expired stock. Lead times for products are increased due to low-volume production runs and the changeover processes between products. The potential for loss in quality increases in smaller production runs because of the complex assemblies and customized parts. Finally, cost...
Sponsored Content
Since 1987, Prometic Bioseparations Ltd. (PBL) has been pioneering the design, development, and manufacture of affinity purification technology for laboratory-scale and industrial-scale bioprocessing.
With 30 years’ experience in the development of affinity products and design and manufacture of new custom adsorbents, PBL is a world leader in its field.
PBL offers an extensive range of off-the-shelf bioseparation products for the recovery and purification of biologicals and the removal of problem contaminants. We also offer a range of semidisposable bioprocess columns (Evolve™ columns) as well as a variety of custom development solutions.
Login to read the full PDF of the application note.
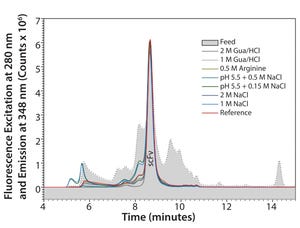
Single-chain variable fragments (scFv) are antibodyderived molecules with several advantages over full-length IgGs, including rapid target access and good tissue penetration. Straightforward and efficient capturing solutions similar to protein A for monoclonal antibodies (mAbs) may pave the way for the future success of this class of molecules. Herein we describe general conditions for capturing scFv from
Escherichia coli
with TOYOPEARL® AF-rProtein L-650F and subsequent optimization to achieve efficient purification.
General Guidelines for CapturinG The κ light chains of mAbs, Fabs, and scFv bind to protein L at neutral pH and physiological conductivity, whereas host-cell proteins (HCPs) flow through. The bound product can then be recovered at pH 2.0–3.5. Generic conditions developed for TOYOPEARL AF-rProtein L-650F are described in Table 1.
Login to read the full PDF of the application note.
Sponsored Content
AbbVie is a global, research-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions. The company’s mission is to use its expertise, dedicated people, and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology, and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world.
Combining the focus of a biotech with the expertise of an established pharmaceutical leader, the contract manufacturing business is agile and flexible in its service to companies outsourcing the development and manufacture of drugs.
Login to read the full PDF of the application note.
Althea is a fully integrated contract development and manufacturing organization providing clinical and commercial drug product services. Althea offers cGMP drug product filling in both vials and syringes and production of microbial-derived recombinant proteins and plasmid DNA. Comprehensive development services are available, including upstream and downstream process development, analytical development, lyophilization, complex formulation, product release, and ICH-compliant stability testing. Althea offers a proprietary formulation technology platform, Crystalomics®, and Corynex® protein expression technology.
Login to read the full PDF of the application note.
Sponsored Content
Avid Bioservices is a contract development and manufacturing organization (CDMO) specializing in mammalian cell culture process development and cGMP production of clinical and commercial-scale monoclonal antibodies, recombinant proteins, and enzymes. Committed to the success of our clients, our team constantly strives to build partnerships that extend well beyond the delivery of your product. By combining our knowledge and experience with a flexible and efficient approach, we can meet your specific project requirements.
Established CDMO with a Broad Range of Capabilities
Proven Track Record in CGMP Biologics Manufacturing
Login to read the full PDF of the application note.
The analytical field for biologics has evolved greatly over the past 30 years, and the underlying growth has shifted from biopharmaceutical companies to contract research organizations (CROs). The global biopharmaceutical market is growing annually at >15%, making it the largest and consistently fastest growing segment of the healthcare industry with annual sales in excess of US$200 billion. Contract manufacturing organizations (CMOs) are expanding capacity by building new cost-efficient facilities, reflecting market demand. Many product sponsors are outsourcing, some even increasing their outsourced work.
Meanwhile, the pharmaceutical industry is consolidating through acquisitions and mergers as well as forming strategic alliances. That represents a minor threat to some CMOs by shrinking their client market. Currently the CMO market is very fragmented, with hundreds of companies across the globe — and it also is undergoing some consolidation, which is key to improving profitability. Late in 2015, the tot...
Our state-of-the-art biologics manufacturing facility (which uses single-use systems) is expanding to add 22,000 ft
2
of space and with that, the flexibility and scale to support your growth. The expansion adds a new 2 × 2,000-L single-use bioreactor system to support late-phase clinical and commercial-scale production of up to 4,000-L batches, as well as expansion of analytical and process development laboratories. Catalent offers a broad range of integrated process development and analytical services to solve your most difficult development and biologics clinical and commercial manufacturing challenges, including
Login to read the full PDF of the application note.
The quality of pharmaceutical products is the top priority for both biopharmaceutical companies and regulators. To ensure consistently high product quality and improve the efficiency of manufacturing and regulation, the US FDA introduced quality by design (QbD) to the pharmaceutical industry in its 2002 Pharmaceutical CGMP initiative,
Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach
(
1
).
Since the introduction of QbD, the FDA has taken multiple measures to promote industry-wide implementation, but the industry’s response to QbD has been mixed. Although many biopharmaceutical companies fully embrace the concept, incorporating QbD approach in their development programs, many others remain hesitant to adopt QbD and thoroughly integrate its principles into their operations. Conventional wisdom says it is better to act now and gain practical experience with QbD before regulators mandate their wholesale adoption.
Login to read the full PDF of the application note.
Reference:
1
Pharmaceutic...