Introduction
by William P. Flanagan
Biopharmaceutical development and manufacturing demand scalable processes that can be quickly developed, easily implemented, and smoothly transferred to production. Disposable, ready-to-use technologies play a crucial role in providing flexibility to support agile biomanufacturing operations. Single-use systems provide process efficiencies by removing steps such as cleaning and cleaning validation, thus allowing for faster change-over between manufacturing runs.
The biopharmaceutical industry is increasingly adopting single-use approaches, and the global market for such bioprocessing tools is expected to grow at a compound annual growth rate of 11.7% through 2019 (
1
). Meanwhile, the world continues to face mounting pressure related to a variety of environmental concerns: climate, water scarcity, and other resource availability issues. Rapid shifts in technology development or adoption present an opportunity — and an obligation — to evaluate sustainability implication...
New Sandoz Biosimilar Drug-Product Facility
Workers in a compounding room at the new Sandoz facility
In September 2015, Sandoz (the generics and biosimilars business of Swiss drug maker Novartis) opened its BioInject biomanufacturing facility in Schaftenau, Austria. Over €150 million of investment is creating 100 highly skilled jobs in a state-of-the-art facility for manufacturing prefilled syringes and devices for both biosimilars and novel biologics. Staff can fill 18,000 syringes/hour and package 100 syringes/minute.
In welcoming guests at the opening, Carol Lynch (global head of biopharmaceuticals and oncology injectables at Sandoz) said that BioInject will be central to a Novartis biomanufacturing network that includes facilities in Slovenia, Singapore, and France. The company has invested €2.2 billion in Austria since 1996, with more than 4,600 employees at four Austrian sites.
In September, Sandoz became the first company to introduce a biosimilar for the US market. It has several biosimilars in di...
The BioProcess International Conference took place during the last week in October in Boston, MA — when technical editor Cheryl Scott and I enjoyed meeting with so many of our readers, authors, and advisors as well as the BPI sales staff. We always receive good suggestions from our editorial advisors in our annual meeting with them, and the technical presentations help us craft the final version of our 2016 editorial calendar. As always, congratulations to our Informa colleagues at IBC for another excellent program.
This December issue is our fourth annual departure from the usual more technical content. Here we look at a number of business and bigger-pictures issues that affect your work in developing biopharmaceuticals. Authors in this issue touch upon key topics including those affecting progress in single-use technologies, biosimilar development, information technology, decision-making, product portfolios and planning, and cross-team management.
Our fall issues (based on the familiar alternating produ...
WWW.GRAPHICSTOCK.COM
Johnson & Johnson’s pharmaceutical research arm recently launched a drug-development project that will intercept diseases before patients even realize they’re sick. In the company’s words: “The future of healthcare will increasingly depend on identifying and correctly interpreting the earliest signals of disease susceptibility, preventing or intercepting disease before it even begins.” (
1
).
The first targets include Alzheimer’s, some forms of cancer, heart disease, rheumatoid arthritis, multiple sclerosis, and type 1 diabetes. The company’s Disease Interception Accelerator group will search for genetic variations and other molecular factors that are the root causes of diseases and that can be detected long before clinical symptoms materialize. Other groups will develop treatments that block or halt their initiation or reverse their progress very early in their development. Steve Brozak, president of WBB Securities, estimates that accomplishing these stated goals will cost billions o...
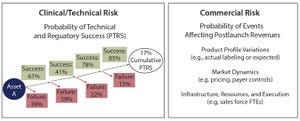
Figure 1: Sources of risk for portfolio assets
In the biopharmaceutical industry, portfolio risk is categorized as clinical/technical or commercial (Figure 1). Both types pose challenges to quantify. Portfolio managers tasked with optimizing the mix of products in a company’s pipeline often struggle to create apples-to-apples frameworks that can consistently compare risk across asset categories. The framework described herein can help you account for both technical and commercial risk using simple analytical tools.
The foundational analytical element of portfolio management is traditionally the asset forecast: a composite best estimate of the most likely product profile. It is an assessment of the commercial market in which a product will play and is hoped to include some market research to determine that product’s value proposition in the marketplace. Although it is an important exercise in its own right, the forecast alone cannot be the single metric on which portfolio optimization is based. A potential...
GRAPHIC STOCK (WWW.GRAPHICSTOCK.COM)
Like many other industries, life sciences faces rapid, technology-driven changes and a shifting business environment. Many patients are covered by Medicare, Medicaid, or national healthcare organizations outside the United States. That necessitates pricing and reimbursement negotiations that are complicated by rules and regulations. Patients whose health plans belong under a managed-care organization (MCO) or pharmacy-benefit manager (PBM) have access to prescriptions primarily through staff-model pharmacies, mail-order pharmacies, and network pharmacies. Drug manufacturers offer incentives to such organizations in the form of rebates based on the formulary position of a given medication on a health plan.
As contractual commitments with PBMs and MCOs become increasingly complicated — often involving billions of dollars — companies cannot operate with inefficient contract processes. Minor oversights in the initial stages of paper-contract development and negotiations of...
Modern hybrid single-use facility (purification suite)
Recent articles have described how the debate on standardization is slowing down adoption of single-use technology (
1
). The Standardized Disposables Design (SDD) initiative is working to design simple standard single-use solutions for real-life examples (e.g., buffer bags). In reality, a buffer is a buffer whether it is made in Europe, Asia, or America, so in essence different solutions are not necessary for different end users. A buffer bag is not difficult to design, and it does not vary greatly in that design among suppliers and end users.
PM Group, a global engineering design firm, has created a database of ~250 simple assembly designs that can be made by multiple suppliers. These designs can be implemented by end users and engineering firms and can be made available through supplier websites. PM Group does not dictate the fine detail of how bags are manufactured, but rather concentrates on connectivity and end-user strategy.
Assurance of Supply
WWW.GRAPHICSTOCK.COM
Advances in our capabilities for data acquisition, storage, and manipulation are providing the biopharmaceutical industry with an increased understanding of what must be controlled in bioproduction as well as the ability to control it. Developments in hardware, processing algorithms, and software are changing the landscape of bioprocess administration.
Increased power for information gathering and processing began with the remarkable increases in microprocessor speed, pipelining, and parallelism over the past couple of decades (
1
); it continues with advances in data handling and manipulation routines as well as device interfaces (
2
). That foundation supports brilliant new software implementing the highest mathematical, statistical, and logical algorithms. For example GE Healthcare’s UNICORN system control software provides built-in knowledge for planning and controlling runs, and it analyzes results from a number of bioproduction operations. Such advanced software is suitable for ...
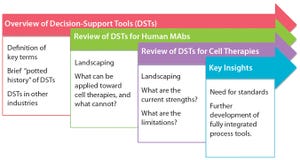
Figure 1: Summary of discussion
Industrial-scale manufacturers in a number of fields — from automobiles to biotherapeutics — have long relied on powerful computational and mathematical tools to aid in the scale-up, optimization, quality control, and monitoring of product development (
1
–
5
). Typical process pathways are highly multifactorial, with numerous branch points, feedback steps, instrumental attributes, and target parameters. Moreover, margins for error are minimal for most industrial processes, requiring high standards of precision from industrial and operational pathways (
6
). For those reasons, the complexity of process engineering and process pathway design necessitates that modeling and decision-support tools (DSTs) be used to ensure high-quality and economically viable end products.
Biologics are no exception to this trend of growing DST use in industrial processes. Compared with classical (small-molecule) pharmaceuticals, biopharmaceuticals exhibit more complex, more expensive, and more ...
WWW.GRAPHICSTOCK.COM
The development pathway of a biosimilar is unlike that of a novel biotherapeutic. Many regulatory authorities reference a stepwise approach to establishing biosimilarity. Analytical requirements are greatly increased before a product enters clinical testing. Enhanced analytical efforts entail physical, chemical, and biological characterization of a biosimilar product compared with an originator reference product. Strategies at this early stage must include intensive characterization of multiple batches to determine variability of quality attributes.
Here I address some issues involved in meeting regulatory characterization expectations by answering the following questions: What regulatory guidelines are associated with structural characterization and comparability/biosimilarity testing? What complicates the characterization of complex (glyco)protein products? When is analytical characterization required? And which techniques (old and new) are suitable for this application?
Evolution o...
The 2009 influenza pandemic was the main driver for the Centers for the Innovation in Advanced Development and Manufacturing (CIADM). As a new federal government authority, the Biomedical Advanced Research and Development Authority (BARDA), directed by Dr. Robin Robinson, assembled a team of scientists and technicians with a vast array of knowledge and experience in chemical, biological, radiation, and nuclear fields. BARDA’s directive was to enhance preparedness for catastrophic challenges such as an influenza pandemic or other serious threats to the American public.
The CIADM has its origins in the
National Strategy for Pandemic Influenza
(
1
). This document has three pillars, with Pillar 1 being “preparedness and communication: to establish domestic production capacity and stockpiles in support of our containment and response strategies,” a high-level perspective. However, the first wave of the 2009 influenza pandemic elevated the recognition that the United States had to be much more prepared for a...