Happy New Year from all of us at BPI. As you can see on the cover, 2017 is the start of our 15th year of publication. Launching a magazine is an uncertain prospect in any era, especially within the trade press format, and our addition of peer-review gives us a split personality. But we are not immune to the print-versus-digital conundrums facing publishers these days, and an anniversary year provides a logical platform on which to update editorial approaches. I have said before that we always default to the needs of our audience, but even among younger, digitally savvy readers, we still meet those who prefer a print product. Is it possible to please everyone — and if so, how? BPI’s editorial calendar for 2017 reveals our plans to approach that ever-elusive goal. They include new editorial products and enhanced connections between print and digital expansions on our product themes.
First, goodbye to our full (issue)-length editorial supplements. We are downsizing them into Featured Reports that will delve ...
New Bioanalytical Testing Laboratory
In November 2016, Sartorius Stedim Biotech (SSB) opened a new bioanalytical and biosafety testing laboratory in the biotech hub area of Boston, MA. This dedicated laboratory is designed to accommodate rising North American demand for the company’s BioOutsource specialized assay platforms and facilitate ongoing expansion of products and testing services. The company performs a range of testing for biologics, vaccines, and biosimilars throughout their development and manufacture.
Reinhardt Vogt is a member of SSB’s executive committee. In welcoming representatives from the biopharmaceutical industry and the local community to this new facility at its opening ceremony, he said it “will offer customers the analytical expertise to help them accelerate entry into clinical phases. We chose Boston as the site for our laboratory because it is a premier biotech hub, enabling us to showcase our portfolio of bioanalytical and biosafety tests, as well as attract the best technical ...
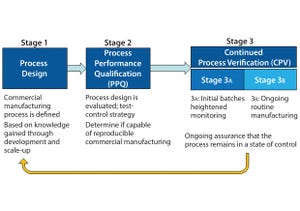
Figure 1: The three stages of process validation as defined by FDA’s 2011 guidance for industry on “Process Validation: General Principles and Practices”
As defined in the ICH Q10 guideline, a
control strategy
is “a planned set of controls, derived from current product and process understanding, that assures process performance and product quality” (
1
). Every biopharmaceutical manufacturing process has an associated control strategy.
FDA’s 2011 guidance for process validation (
2
) describes process validation activities in three stages (Figure 1). A primary goal of stage 1 is to establish a strategy for process control that ensures a commercial process consistently produces acceptable quality products. Biopharmaceutical development culminates in the commercial control strategy, a comprehensive package including analytical and process controls and procedures. Stage 2 process performance qualification (PPQ) is needed to establish scientific evidence that a process is reproducible and will consistently ...
MICAH YOUELLO (WWW.ISTOCKPHOTO.COM)
The BioPhorum Operation Group’s (BPOG’s) Container Closure Integrity Testing (CCIT) workstream would like to congratulate the United States Pharmacopeia’s committee for its latest revision to USP chapter <1207> Package Integrity Evaluation: Sterile Products. Generally, we believe it provides a comprehensive overview of the available methods for container–closure testing and outlines many important elements for consideration in establishing a successful CCIT strategy. We first responded to the USP <1207> draft when it was released for comment in 2014. And from our perspective, some of the changes that were proposed and concerns that were raised in 2014 clearly have been addressed. We are grateful to see that.
The purpose of this letter, however, is to highlight specific areas that cause us concern as a crossindustry group. We are aware that USP’s “informational” chapters are not compulsory. As end-users of such guidances, though — and as representatives of companies that...
WWW.GRAPHICSTOCK.COM
Biologics, primarily therapeutic antibodies and recombinant proteins, represent a growing share of the current drug market, with projected worldwide sales of about US$278 billion by 2020, partly because of their relatively high costs. Indeed, AbbVie’s therapeutic antibody Humira (adalimumab) is currently the top-selling drug in the world by sales but not even in the top 50 by the number of monthly prescriptions. Although the process for marketing generic versions of small molecules under the Hatch–Waxman Act is well understood, the process for marketing biosimilars under the Biologics Price Competition and Innovation Act (BPCIA) still is being established and presents many unresolved questions.
Marketing biosimilars involves a very complex patent litigation procedure that potentially discourages biosimilar development, particularly while the courts are still determining issues left unresolved by Congress and the US Food and Drug Administration (FDA). As an alternative (or supplement) ...
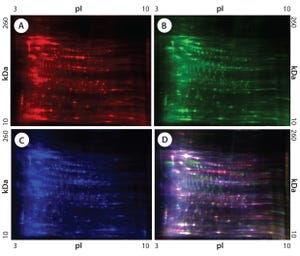
Figure 1: 2-D difference gel electrophoresis (DIGE) analysis of CHO DG44, BioWa CHO, and CHO K1 SV HCPs; (A) CHO DG44 HCP labeled with Cy5, red; (B) BioWa CHO labeled with Cy3, green; (C) CHO K1 SV labeled with Cy2, blue; (D) overlay image of Cy5, Cy3, and Cy2; the range of white to purple represents spots that are common to all three cell lines.
Cell lines derived from Chinese hamster ovary (CHO) cells are widely used in therapeutic protein production because they can perform human-compatible posttranslational modifications, they are easy to use for manufacturing, and they do not propagate most human pathogenic viruses (
1
,
2
). Expressed therapeutic proteins are secreted into CHO culture supernatant along with impurities originating from the host cells themselves. Such host cell proteins (HCPs) are important contaminants for monitoring because they directly affect drug quality, safety, and efficacy.
HCPs are complex mixtures with diverse physicochemical and immunological properties (
3
). The genome s...
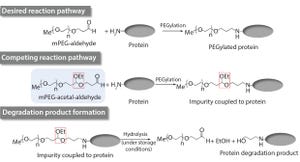
Figure 1: Proposed mechanism for the formation of the drug substance degradation product (
2
)
Impurities related to raw materials used for bioproduction can be inadvertently introduced into a manufacturing process, causing potential failure to meet in-process controls or release specifications. Unexpected impurities also can reduce yield and affect the quality, safety, and effectiveness of a final product (
1
). Raw-material impurities can originate from starting components or reagents used in manufacture. They can be generated in situ during synthesis or as degradation products. Impurities also can result from improper handling, packaging, and storage.
Identification and characterization of such impurities can provide insight into the mechanism of formation for raw material impurities as well as their potential impacts on final drug products (
2
). Such knowledge can be used further for the development of control strategies (e.g., incoming raw material release specifications) to ensure consistent proces...
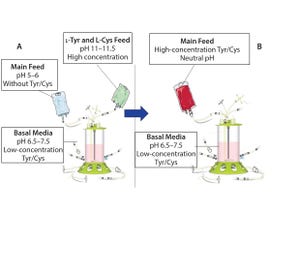
Figure 1: Traditional feeding strategies rely on a main feed and a separate alkaline feed containing l-tyrosine and l-cysteine (A). This study demonstrates the use of a combined main feed with high concentrations of tyrosine and cysteine derivatives at neutral pH (B).
Chinese hamster ovary (CHO) cells commonly are used to produce recombinant proteins such as monoclonal antibodies (MAbs) for research, diagnostic, and therapeutic purposes. Culture processes typically rely on a fed-batch approach in which a basal medium enables initial cell growth. Concentrated feeds are used to prevent nutrient depletion, thereby extending culture duration and improving cell growth, viability, and protein titer.
A neutral pH feed is desirable because culture pH should remain stable after feedings. The extremely low solubility of l-tyrosine and the low stability of l-cysteine at neutral pH have necessitated processes that leverage a slight acidic main feed and a separate alkaline feed containing these essential amino acids. ...
Two years ago, the companies involved in the BioPhorum Operations Group (BPOG) fill–finish community agreed that the quality of elastomer stoppers for vials was causing problems for biopharmaceutical manufacturers. So they deemed it to be a priority for the group. The problem is particularly pronounced for vial stoppers used in legacy products, which may have been on the market for several years. Many such medicines remain valuable for large patient populations. The stoppers used on legacy medicines are manufactured using formulations that do not benefit from modern developments and/or manufacturing methods. The cost of switching to more modern stoppers is perceived to be too high to be a viable option. Long lead times because of registration requirements in many markets create differing regulatory challenges. Stoppers used for legacy products are referred to herein as
legacy stoppers
.
The member companies formed a team “to identify the quality levels that participating organizations require in their st...
with Sharyn Farnsworth, MSc
Scale-up and manufacturing strategy for insect cell culture (ICC) should be assessed at the earliest stages of process development. Your final goals will shape the process and determine at what stage to implement steps that can make the process more reliable and robust at manufacturing scale. Only a few vendors are experienced enough to guide you through them. On 7 December 2016, Sharyn Farnsworth (associate principal scientist at Fujifilm Diosynth Biotechnologies) discussed some upstream challenges BEVS/ICC in a BPI webinar.
Farnsworth’s Presentation
The process we develop is an ICC using Sf21, Sf9, and High-Five cell lines to produce recombinant proteins. The BEVS delivers a gene of interest into cells. More than 500 baculoviruses can infect and produce recombinant proteins in insect cells. This system can make very large molecules at high concentrations — e.g., virus-like proteins (VLPs) for vaccines. It’s also capable of certain posttranslational modifications, but not to t...